Oxidation and Reduction
This lesson covers:
- Identifying redox reactions
- Using oxidation numbers to identify redox reactions
- Oxidising agents and reducing agents
- Redox reactions between metals and acids
Redox involves electron transfer
Oxidation is the process of electron loss. Reduction is the process of electron gain.
These processes occur simultaneously, which is why they are referred to collectively as a "redox reaction".
- An oxidising agent accepts electrons and undergoes reduction itself.
- A reducing agent donates electrons and undergoes oxidation itself.
For example, consider the reaction between sodium and chlorine to form sodium chloride:
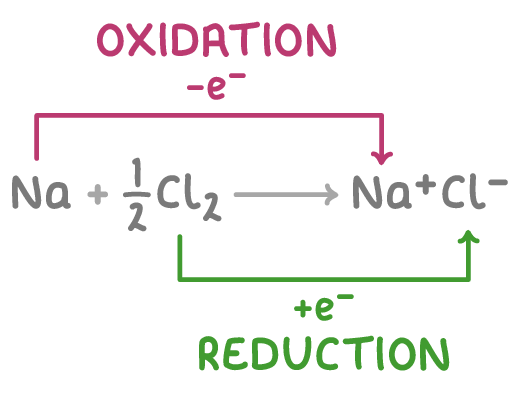
- Sodium is oxidised because it loses electrons, acting as the reducing agent.
- Chlorine is reduced because it gains electrons, acting as the oxidising agent.
Oxidation numbers change in redox reactions
Oxidation numbers increase by 1 for every electron lost.
Oxidation numbers decrease by 1 for every electron gained.
To determine if a redox reaction has occurred, compare the oxidation numbers before and after the reaction:
- An increase indicates oxidation
- A decrease indicates reduction
For example, in the reaction:
Na(s) + 1⁄2Cl2(g) ➔ NaCl(s)
- Sodium is oxidised because its oxidation number increases from 0 (in Na) to +1 (in NaCl).
- Chlorine is reduced because its oxidation number decreases from 0 (in Cl2) to -1 (in NaCl).
The mnemonic OILRIG can help you remember that oxidation is a loss of electrons and reduction is a gain of electrons:
Oxidation
Is
Loss
Reduction
Is
Gain
Redox reactions occur between metals and acids
A reaction between a metal and an acid to produce a salt and hydrogen gas exemplifies a redox reaction:
- Metal atoms lose electrons and are oxidised
- Hydrogen ions gain electrons and are reduced
For example, in the reaction:
Mg(s) + 2HCl(aq) ➔ MgCl2(aq) + H2(g)
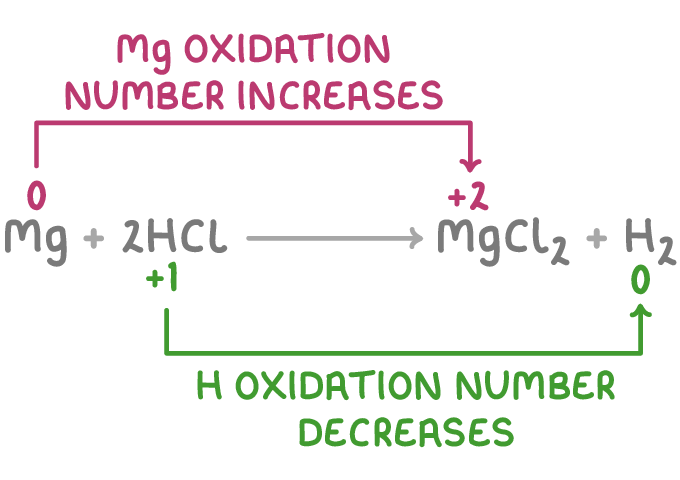
- Magnesium is oxidised because its oxidation number increases from 0 to +2
- Hydrogen ions are reduced because their oxidation number decreases from +1 to 0.
- Chloride ions are neither oxidised nor reduced because their oxidation number remains unchanged. Any species whose oxidation number remains constant throughout the reaction acts as a spectator ion and is not included in the ionic equation:
Mg(s) + 2H+(aq) ➔ Mg2+(aq) + H2(g)