Esters
This lesson covers:
- The structure and naming of esters
- Uses of esters
- Methods to make esters
- Hydrolysis of esters
- The difference between fats and oils
- Making soap and biodiesel
Esters contain the functional group -COO-
Esters are organic compounds formed from the reaction between a carboxylic acid and an alcohol, where a molecule of water is removed during the process. The general structure of an ester is R-COO-R’.
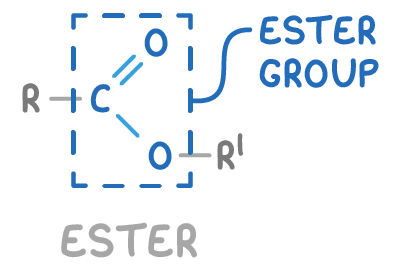
Where:
- R is an alkyl or aryl group from the carboxylic acid.
- COO- is the ester functional group.
- R’ is an alkyl or aryl group from the alcohol.
Naming esters
The name of an ester consists of two parts:
- The first part comes from the alcohol used and is the alkyl (R) group.
- The second part comes from the carboxylic acid used. The acid ending is replaced by 'oate' to give the second part of the name.
For example, propyl ethanoate is formed from propanol and ethanoic acid:
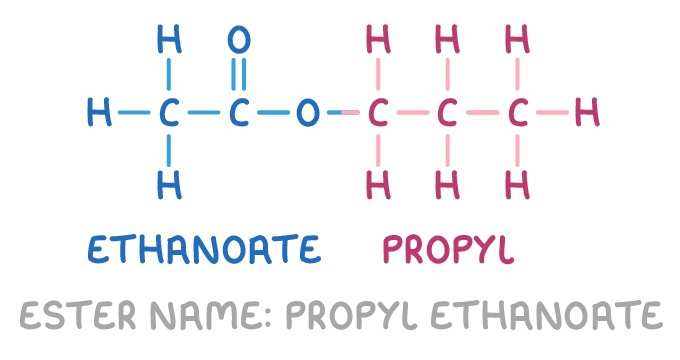
Uses of esters
Esters have several commercial uses:
- Flavourings and perfumes - Due to their often sweet, fruity scents, esters are popular in the food and fragrance industries.
- Solvents - They are effective in dissolving other organic substances and have relatively low boiling points, making them suitable for use in chemical processes and manufacturing.
- Plasticisers - By incorporating them into plastics, they enhance flexibility. However, over time, these plasticisers may evaporate, causing the plastic to become brittle.
Synthesising esters
There are primarily three methods for synthesising esters.
1. From alcohols and carboxylic acids
- Mix the carboxylic acid and alcohol under reflux with an acid catalyst, such as H2SO4, in a reaction known as esterification.
- Applying an excess of alcohol can drive the reaction towards the formation of the ester product.
- The resulting ester can be isolated using fractional distillation.
- For example, the reaction to produce ethyl ethanoate from ethanol and ethanoic acid is:
C2H5OH + CH3COOH ⇌ CH3COOC2H5 + H2O
2. From alcohols and acid anhydrides
- Combine the acid anhydride with alcohol at a warm temperature to produce an ester and a carboxylic acid.
- Fractional distillation is used for separation.
- For example, the reaction to produce ethyl ethanoate from ethanol and ethanoic anhydride is:
C2H5OH + (CH3CO)2O ➔ CH3COOC2H5 + CH3COOH
3. From alcohols and acyl chlorides
- Mixing acyl chloride with alcohol at room temperature leads to a rapid and vigorous reaction, producing an ester and hydrochloric acid gas.
- For example, the reaction to produce ethyl ethanoate from ethanol and ethanoyl chloride is:
C2H5OH + CH3COCl ➔ CH3COOC2H5 + HCl
Hydrolysis of esters
The process of hydrolysis breaks down esters into their original alcohol and carboxylic acid components using water.
There are two hydrolysis methods:
- Acid hydrolysis - The ester is heated under reflux with a dilute acid, such as HCl or H2SO4, to produce a carboxylic acid and an alcohol.
The general equation is: RCOOR’ + H2O ⇌ RCOOH + R’OH
- Base hydrolysis - When the ester is heated under reflux with a dilute base like NaOH, the products are a carboxylate salt and alcohol. This method is generally irreversible due to the stability of the carboxylate salt.
The general equation is: RCOOR’ + NaOH ➔ RCOONa + R’OH
Comparing fats and oils
Animal fats and vegatable oils are esters made from the alcohol glycerol (propane-1,2,3-triol) and long chain fatty acids. Specifically, they are triglycerides - esters containing three fatty acid chains attached to a glycerol backbone via ester linkage.
The general structure of a triglyceride is:
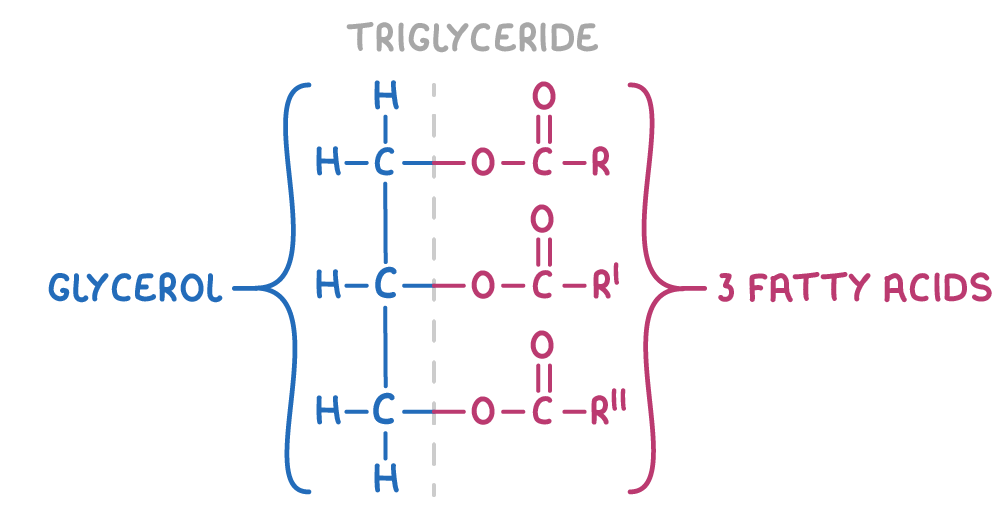
Differences between fats and oils
Whether a triglyceride is a solid fat or liquid oil depends on the structure of its fatty acid chains and the resulting intermolecular forces:
- Saturated fatty acids - Found in animal fats, these contain only single carbon-carbon bonds, resulting in straight hydrocarbon chains. The straight chains allow tight packing of fat molecules, enabling stronger intermolecular forces between them. These stronger forces raise their melting point, causing them to be solid at room temperature.
- Unsaturated fatty acids - Found in vegetable oils, these contain carbon-carbon double bonds which cause kinks or bends in the hydrocarbon chains. The bent shapes prevent close packing of oil molecules, resulting in weaker intermolecular forces. These weaker forces lower their melting point, allowing them to remain liquid at room temperature.
Making soap
Soap is produced by hydrolysing fats and oils through boiling with a strong alkali solution, such as sodium hydroxide. This reaction breaks down the ester bonds in triglycerides, forming glycerol and sodium salts of the fatty acids. These sodium salts make up the soap.
The general reaction is:
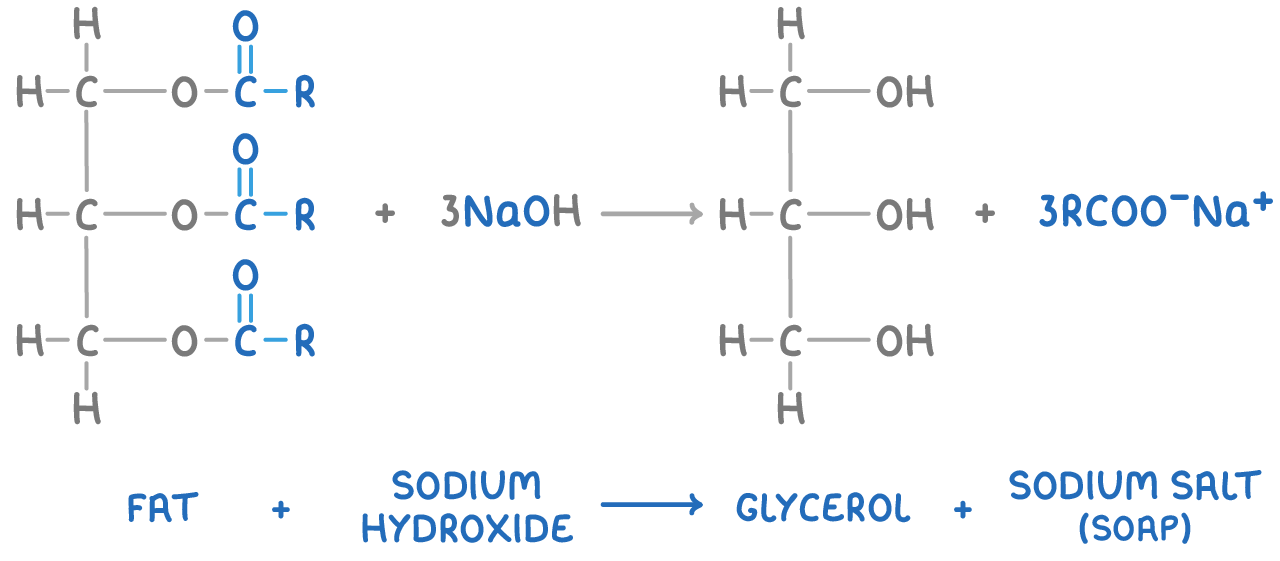
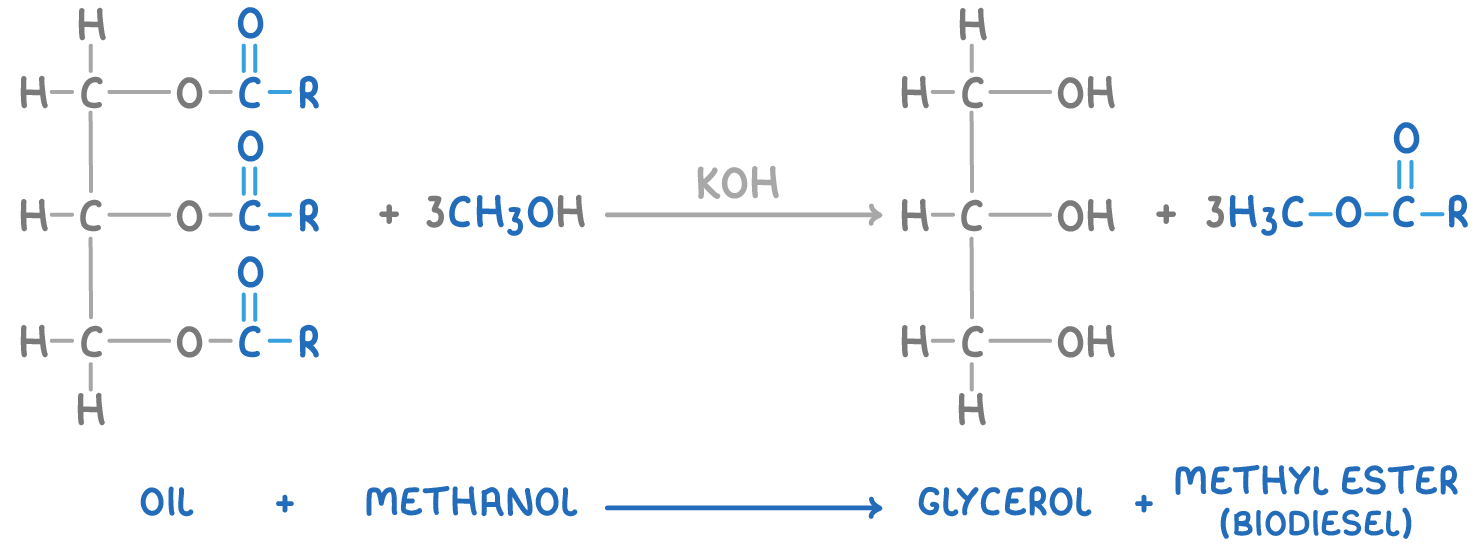
Making biodiesel
Biodiesel is produced by reacting vegetable oils with methanol in the presence of a potassium hydroxide catalyst. This reaction leads to the formation of glycerol and methyl esters of fatty acids, which serve as effective substitutes for diesel fuel.
The general reaction is:
Biodiesel is considered a renewable fuel because it is derived from plant-based oils that can be replenished relatively quickly through agricultural processes, unlike fossil fuels that take millions of years to form. As a result, biodiesel offers a more environmentally friendly alternative to traditional fossil fuels.