Ethanol Production
This lesson covers:
- Industrial ethanol production by hydration of ethene
- Industrial ethanol production by fermentation of glucose
- Ethanol as a biofuel
- Carbon neutrality of bioethanol
Industrial ethanol production by hydration of ethene
Ethanol can be produced industrially through the chemical reaction known as hydration, where water (H2O) is added to ethene (C2H4) in the presence of an acid catalyst. Ethene is typically obtained from the cracking of crude oil, a process that breaks down larger hydrocarbon molecules into smaller, more useful ones.
The hydration reaction is as follows:
C2H4(g) + H2O(g) ➔ C2H5OH(l)
The reaction mechanism involves three distinct stages:
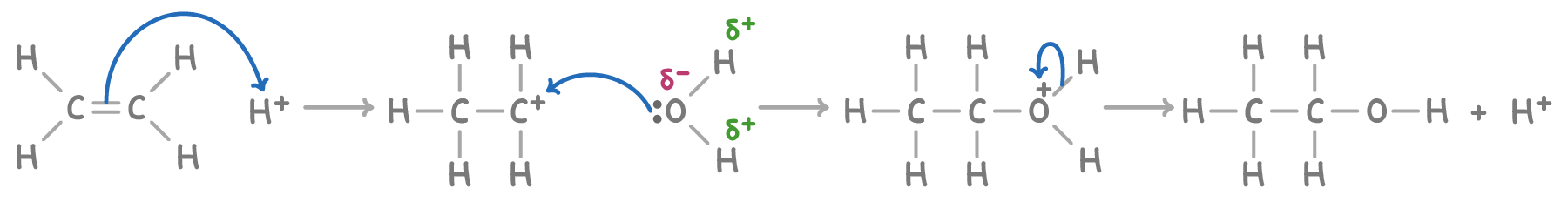
- The formation of a carbocation occurs when the pi bond of ethene breaks and a proton (H+) attaches to one of the carbon atoms.
- A water molecule then attaches itself to the positively charged carbocation.
- Finally, a proton (H+) is lost from the newly attached water molecule, resulting in the formation of the ethanol molecule.
Industrially, this reaction is typically carried out by mixing ethene with steam at a temperature of 300°C and a pressure of 60 atm, using solid phosphoric(V) acid as the catalyst.
Industrial ethanol production by fermentation
Another method for producing ethanol on an industrial scale is through the fermentation of glucose by yeast, described by the equation:
C6H12O6(aq) ➔ 2C2H5OH(aq) + 2CO2(g)
In the fermentation reaction, yeast enzymes break down glucose into ethanol and carbon dioxide. This process occurs anaerobically (without oxygen) at 30-40°C.
- Anaerobic conditions are used to ensure yeast performs anaerobic respiration. If oxygen was present, yeast would respire aerobically instead, producing ethanoic acid instead of ethanol.
- A temperature of 30-40°C is used because this is the optimal range for the yeast enzymes to function. Temperatures above 40°C would begin to denature the enzymes.
Following fermentation, the ethanol is separated from the mixture through fractional distillation. Despite its reliance on renewable resources, the additional steps required for purification currently make fermentation a more costly method compared to the hydration of ethene.
Ethanol as a biofuel
Biofuels are renewable fuels derived from recently living biological materials, such as plants, algae, or organic waste. Ethanol produced via fermentation, also known as bioethanol, is a biofuel that can be used as an alternative to or as a supplement to petrol in transportation.
Advantages of bioethanol:
- Renewable - Produced from crops that can be replanted and harvested repeatedly.
- Lower net CO2 emissions - The crops used for ethanol production absorb CO2 from the atmosphere during their growth phase, which can offset some of the emissions from fuel use.
Disadvantages of bioethanol:
1. Engine modifications - Some engines may require adjustments to efficiently use fuels with high ethanol content.
2. Competition for land - Using agricultural land for fuel production can reduce the area available for food crops.
Is ethanol fermentation carbon neutral?
The notion of carbon neutrality in bioethanol production hinges on the cycle of CO2 absorption and emission:
- Plants absorb CO2 from the atmosphere during photosynthesis.
- The CO2 is then released back into the atmosphere when the ethanol produced is burned as fuel.
The equations demonstrate that six moles of CO2 are absorbed during photosynthesis, matching the six moles of CO2 later released in the fermentation and combustion stages.
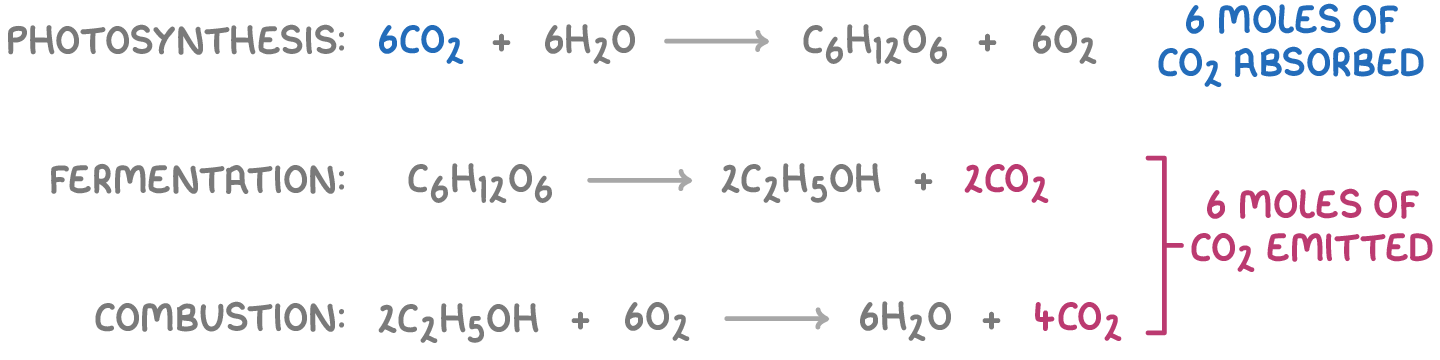
This cycle suggests a balance between the CO2 absorbed and emitted.
However, the overall process is not entirely carbon neutral due to:
- The fossil fuels used to produce fertilisers for the crops.
- The energy consumed by farm machinery during planting and harvesting.
- The transportation of both the feedstock and the final ethanol product.
These activities contribute additional CO2 emissions, indicating that while the fermentation process recycles CO2, it does not eliminate the reliance on fossil fuels entirely. Therefore, ethanol production through fermentation, while beneficial in reducing some carbon emissions, cannot be considered completely carbon neutral.