Forces Acting Between Molecules
This lesson covers:
- The types of intermolecular force
- Induced dipole-dipole forces
- Permanent dipole-dipole forces
- Hydrogen bonding
- How intermolecular forces affect the properties of substances
Intermolecular forces are weak attractions between molecules
Intermolecular forces are the attractive forces that exist between molecules. These forces are much weaker than the covalent bonds that hold atoms together within molecules.
There are three main types of intermolecular force, listed in order of increasing strength:
- Induced dipole-dipole forces (also known as London dispersion forces or Van der Waals’ forces)
- Permanent dipole-dipole forces
- Hydrogen bonding
The key characteristics of these three types of intermolecular force are summarised in the table below:
| Name of intermolecular force | Strength | Found in |
|---|---|---|
| Induced dipole-dipole forces | Weak | All molecules and noble gases |
| Permanent dipole-dipole forces | Moderate | Polar molecules |
| Hydrogen bonding | Strong | Polar molecules with H-F, H-O or H-N bond |
Induced dipole-dipole forces occur between all molecules
Induced dipole-dipole forces, also known as London dispersion forces or Van der Waals’ forces, are present between all atoms and molecules, even non-polar ones. They arise due to temporary fluctuations in the electron distribution around atoms:
How induced dipole-dipole forces arise
- Electrons in atoms are constantly moving. At any instant, there may be more electrons on one side of the atom than the other, creating a temporary dipole.
- This temporary dipole can induce an opposite dipole in a neighbouring atom, causing a weak electrostatic attraction between the atoms.
- This induced dipole can then induce further dipoles in other nearby particles.
- Although these dipoles are constantly forming and disappearing as the electrons move, the overall effect is a net attraction between the atoms or molecules.
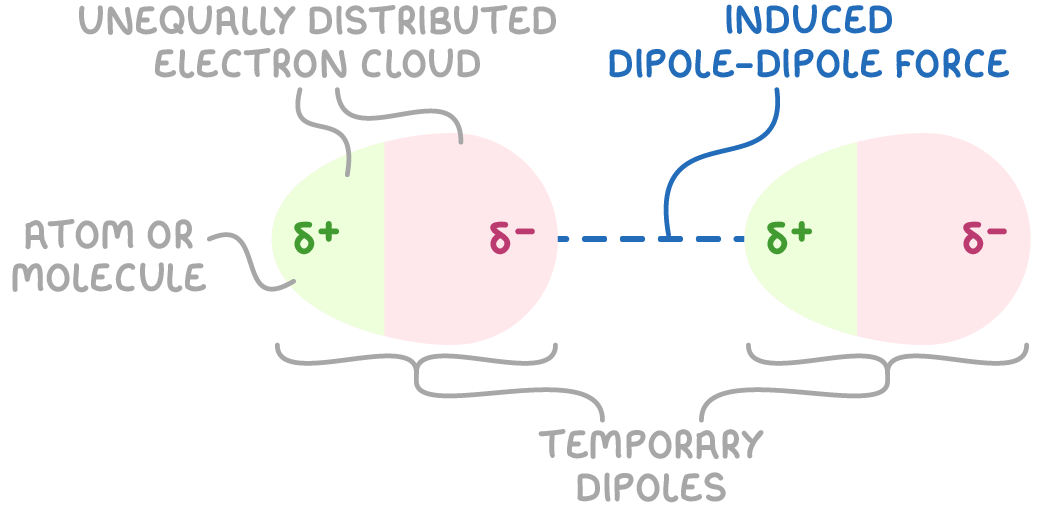
Factors affecting the strength of induced dipole-dipole forces
The strength of induced dipole-dipole forces increases with the size and surface area of the atoms or molecules:
- Size - Larger atoms and molecules have more electrons and a greater volume of electron density that can become polarised, creating stronger temporary dipoles.
- Surface area - Molecules with a larger surface area also have stronger induced dipole-dipole forces as more of the electron cloud is exposed for interactions.
Consequently, substances with stronger induced dipole-dipole forces tend to have higher boiling points.
For example, the boiling points of the group 4 hydrides increase down the group:
| Hydride | Molecular formula | Boiling point (K) |
|---|---|---|
| Methane | CH4 | 111 |
| Silane | SiH4 | 161 |
| Germane | GeH4 | 185 |
| Stannane | SnH4 | 221 |
The trend occurs because molecular size and number of electrons increase down the group, resulting in stronger induced dipole-dipole forces and higher boiling points.
Induced dipole-dipole forces in molecular lattices and noble gases
Induced dipole-dipole forces can also be strong enough to hold molecules together in a lattice structure. For example:
- In solid iodine, I2 molecules are held together by strong covalent bonds.
- These I2 molecules are then attracted to each other by weak induced dipole-dipole forces, forming a molecular lattice.
Induced dipole-dipole forces also explain the existence of noble gas liquids and solids - even though noble gas atoms have complete outer shells and do not form covalent, ionic or metallic bonds, the weak induced dipole-dipole forces allow them to condense into the liquid and solid states at very low temperatures.
Polar molecules experience permanent dipole-dipole forces
Polar molecules have permanent dipoles arising from unequal sharing of electrons in covalent bonds. The partial positive (δ+) and partial negative (δ-) charges on polar molecules enable them to experience permanent dipole-dipole forces.
How permanent dipole-dipole forces arise
Permanent dipole-dipole forces are electrostatic attractions between the partial positive end of one polar molecule and the partial negative end of another.
For example, in gaseous hydrogen chloride (HCl):
- The H-Cl bond is polar due to the greater electronegativity of chlorine compared to hydrogen.
- The hydrogen atom bears a partial positive charge (δ+) and the chlorine a partial negative charge (δ-).
- HCl molecules align so the δ+ hydrogen of one molecule is attracted to the δ- chlorine of a neighbouring molecule.
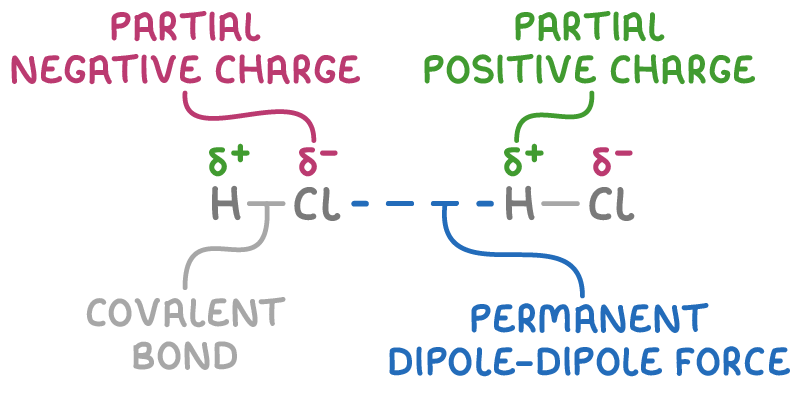
Polar molecules contain permanent and induced dipole-dipole forces
These permanent dipole-dipole forces are in addition to the induced dipole-dipole forces that exist between all molecules. So polar molecules have stronger overall intermolecular forces than non-polar molecules of similar size.
For example, methanal (CH2O) has a higher boiling point than ethane (C2H6):
| Molecule | Molecular formula | Polarity | Boiling point (K) |
|---|---|---|---|
| Methanal | CH2O | Polar | 254 |
| Ethane | C2H6 | Non-polar | 184 |
The difference in boiling points can be explained by the types of intermolecular forces present in each substance:
- Methanal is a polar molecule due to the carbonyl group (C=O), so it experiences both permanent dipole-dipole forces and induced dipole-dipole forces. The permanent dipole-dipole forces are stronger than the induced dipole-dipole forces alone.
- Ethane is a non-polar molecule, so it only experiences induced dipole-dipole forces.
As a result, more energy is required to overcome the stronger intermolecular forces in methanal, leading to a higher boiling point, even though the molecules are of similar size and have the same molecular mass (Mr = 30).
Hydrogen bonding is the strongest type of intermolecular force
Hydrogen bonding is a special type of (permanent) dipole-dipole force that occurs when hydrogen is bonded to the highly electronegative elements fluorine, oxygen or nitrogen.
Requirements for hydrogen bonding
For hydrogen bonding to occur, two criteria must be met:
- The molecule must contain a hydrogen atom covalently bonded to either fluorine (F), oxygen (O), or nitrogen (N).
- There must be a lone pair of electrons on the F, O, or N atom of an adjacent molecule available to interact with the hydrogen.
How hydrogen bonds form
- The H-F, H-O, and H-N bonds are highly polar due to the large electronegativity differences between hydrogen and these elements. This leads to a significant partial positive charge (δ+) on the hydrogen atom and a partial negative charge (δ-) on the F, O, or N atom.
- The small size of the hydrogen atom allows it to get close to the lone pair of electrons on an adjacent F, O, or N atom.
- The lone pairs on F, O, and N atoms are regions of high electron density and therefore high partial negative charge.
- The positively charged hydrogen is strongly attracted to the negatively charged lone pair, forming a hydrogen bond between the molecules.
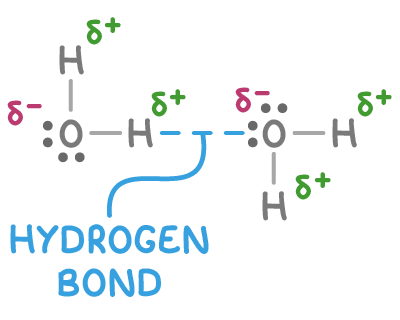
Hydrogen bonding occurs in molecules containing N-H, O-H and H-F bonds, such as water (H2O), ammonia (NH3) and hydrogen fluoride (HF).
Impact of hydrogen bonding on properties
Hydrogen bonding has significant effects on the properties of substances:
- Greater solubility in water - Substances that can form hydrogen bonds with water (e.g., ethanol) tend to be soluble, while those that cannot (e.g., ethane) are typically insoluble.
- Higher melting and boiling points compared to similar-sized molecules that cannot hydrogen bond - Extra energy is needed to overcome the strong hydrogen bonding forces.
For example, consider the boiling points of the group 6 hydrides:
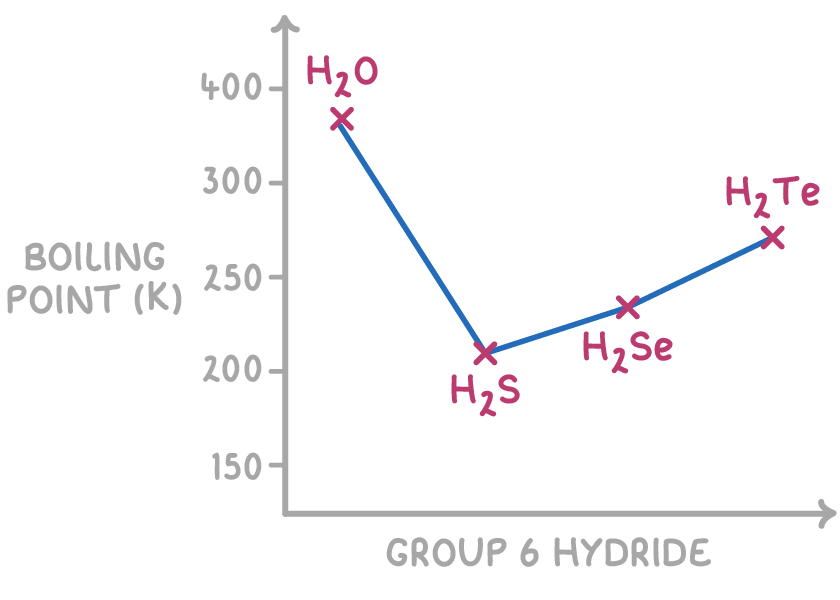
- Water (H2O) has a significantly higher boiling point than the other group 6 hydrides due to its ability to form hydrogen bonds. The strong intermolecular forces created by hydrogen bonding require more energy to overcome, resulting in a higher boiling point.
- The trend of increasing boiling points from H2S to H2Te can be attributed to the increasing molecular mass and size of the molecules. As the molecules become larger, the strength of the induced dipole-dipole forces increases, leading to higher boiling points.
Hydrogen bonding explains the anomalous properties of water and ice
Hydrogen bonding significantly impacts the structure and properties of water and ice:
- Ice is denser than water - In solid ice, water molecules are arranged in a three-dimensional lattice held together by hydrogen bonds. Upon melting, some of these hydrogen bonds break. Since hydrogen bonds are relatively long compared to covalent bonds, this causes ice to be less dense than liquid water.
- Water and ice have high melting and boiling points - Water has relatively high melting and boiling points compared to other molecules of similar size. This is due to the strong hydrogen bonds between H2O molecules, which require more energy to break.
Intermolecular forces explain properties of simple molecular substances
The type and strength of intermolecular forces present in a simple molecular substance influence its physical properties.
- Low melting and boiling points - Weak intermolecular forces require little energy to overcome, so covalent compounds often have low melting and boiling points and may be liquid or gaseous at room temperature. Stronger intermolecular forces lead to higher melting and boiling points.
- Solubility in water - Polar molecules, especially those capable of hydrogen bonding (e.g., ethanoic acid), can interact favorably with water molecules and are soluble. Non-polar molecules that only have induced dipole-dipole forces (e.g., hexane) are hydrophobic and insoluble in water.
- Electrical conductivity - Covalent compounds do not conduct electricity, regardless of polarity. Even though polar molecules have permanent dipoles, they are electrically neutral overall and do not carry charge.