Catalysts
This lesson covers:
- What catalysts are
- How catalysts work
Definition of a catalyst
A catalyst is a substance that increases the rate of a chemical reaction. It does this by providing an alternative route for the reaction that has a lower activation energy. Importantly, the catalyst itself is not consumed in the reaction; it temporarily participates in the reaction but is chemically regenerated at the end.
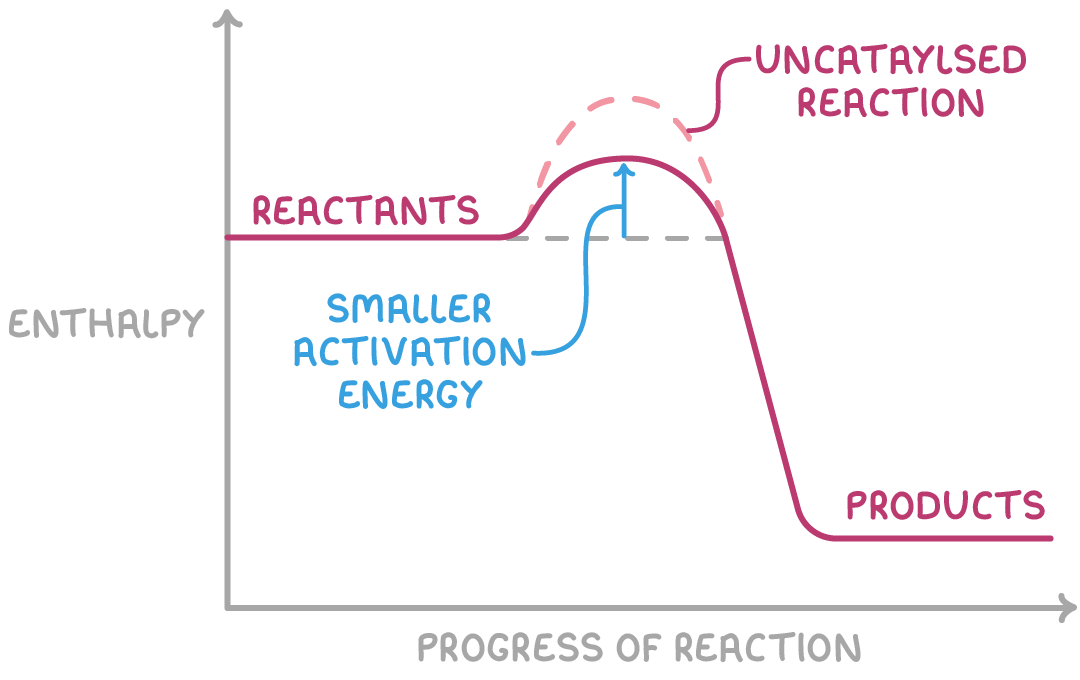
The enthalpy profile diagram above illustrates this concept by showing that the activation energy of the catalysed reaction pathway is lower than that of the uncatalysed reaction pathway.
How catalysts work
Catalysts are specific - they usually work only for particular reactions by interacting with specific reactants.
They provide an alternative pathway for the reaction which has a lower activation energy. This lower energy requirement means a larger number of reactant particles have sufficient energy to undergo the reaction.
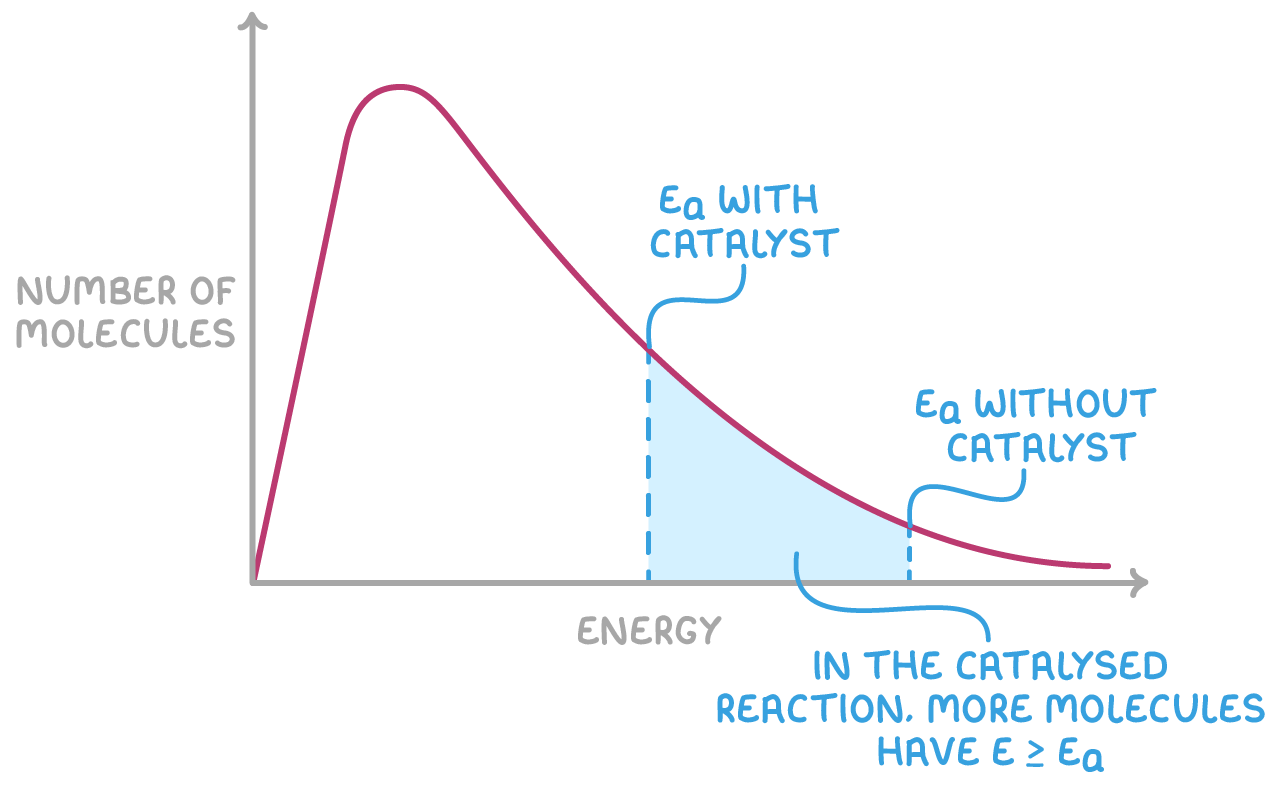
The Maxwell-Boltzmann distribution shows that, at a given temperature, the energies of gas particles are spread over a wide range. By lowering the activation energy, a catalyst increases the fraction of particles that have enough energy to react, thereby increasing the rate of the reaction.