The Shapes of Molecules and Ions
This lesson covers:
- How the number and type of electron pairs determine molecular shape
- Electron pair repulsion theory
- Predicting the geometry of molecules and ions
Molecular shape depends on electron pair arrangement
The three-dimensional shape of a molecule or ion depends on the number and arrangement of electron pairs surrounding its central atom. These electron pairs fall into two categories:
- Bonding pairs - Involved in forming covalent bonds with other atoms.
- Lone pairs - Not involved in bonding and remain on the central atom.
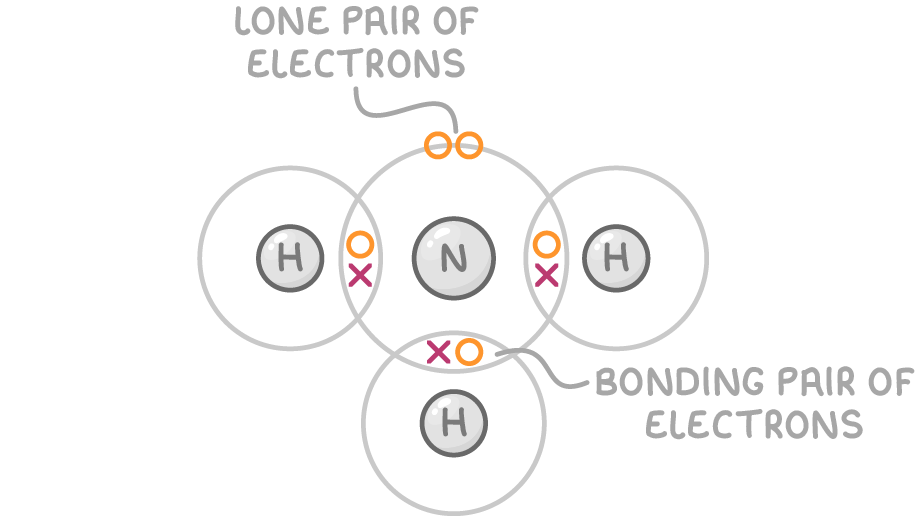
For instance, the ammonia molecule (NH3) has four electron pairs around its central nitrogen atom: three are bonding pairs connected to hydrogen atoms, and one is a lone pair.
Electron pair repulsion theory
A molecule adopts a shape that minimises the repulsion between its electron pairs due to their negative charge:
- Electron pairs, whether bonding or lone, repel each other.
- Lone pairs cause more repulsion than bonding pairs because they are closer to the nucleus.
- Electron pairs arrange themselves as far apart as possible to minimise repulsion.
Repulsion decreases in this order:
Lone pair-lone pair > Lone pair-bonding pair > Bonding pair-bonding pair.
Consequently, lone pairs occupy more space, affecting the molecule's shape by compressing bond angles. This principle underpins the electron pair repulsion theory, which helps predict molecular geometry.
Examples include methane (CH4), ammonia (NH3), and water (H2O), which differ in bond angle and shape despite each having four electron pairs around the central atom due to the varying number of lone pairs.
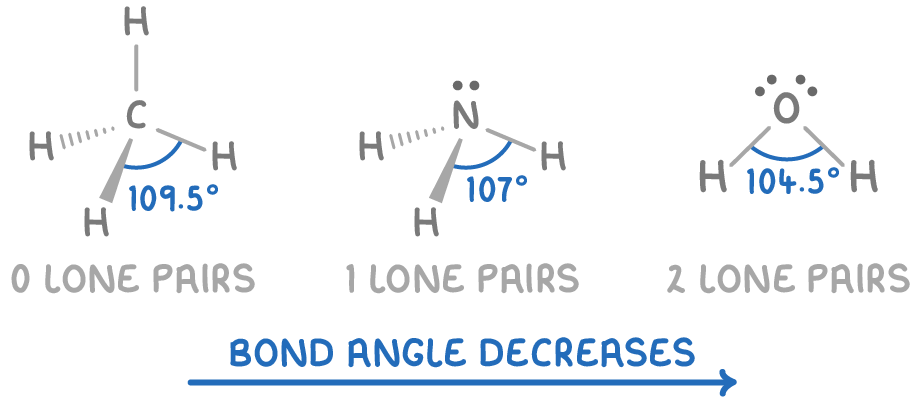
| Molecule | Electron pairs | Bond angle (°) |
|---|---|---|
| CH4 | 4 bonding, 0 lone | 109.5 |
| NH3 | 3 bonding, 1 lone | 107 |
| H2O | 2 bonding, 2 lone | 104.5 |
Determining the number of electron pairs
To predict the shape of a molecule or ion, first determine the total number of electron pairs (bonding + lone) on the central atom using these steps:
- Identify the central atom bonded to all other atoms.
- Find the number of outer shell electrons of the central atom using its group number.
- Add one electron for each bonded atom.
- If the species is an ion, add one electron for each negative charge or subtract one electron for each positive charge.
- Divide the total number of electrons by two to get the number of electron pairs.
- Subtract the number of bonds from the number of electron pairs to determine the number of lone pairs.
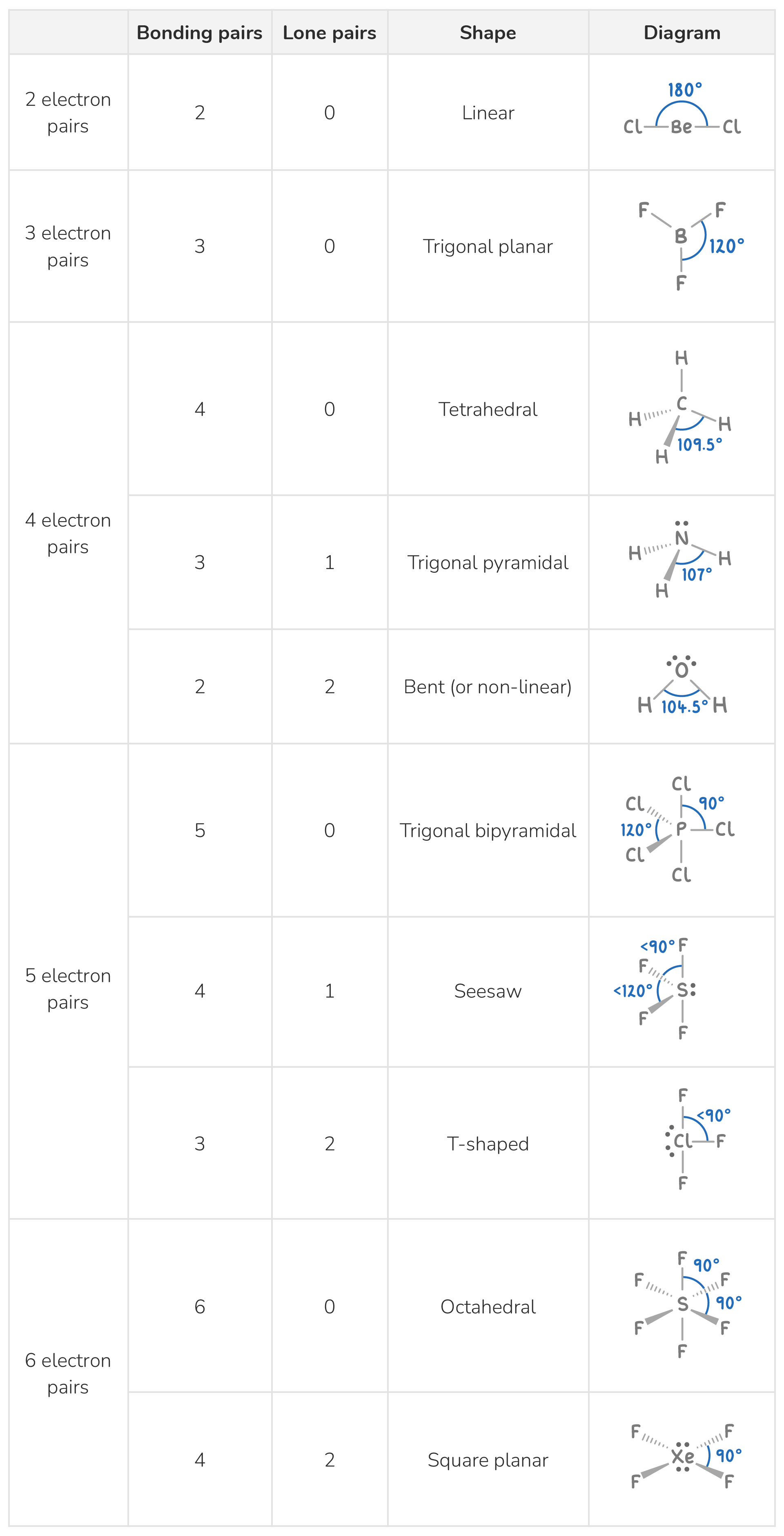
Based on the electron pair arrangement, the molecular shape can be predicted as follows:
Worked example 1 - Predicting the shape of the OF2 molecule
Let's apply the steps to predict the shape of a oxygen difluoride (OF2) molecule.
Step 1: Identify the central atom
Oxygen (O) is the central atom.
Step 2: Calculate total outer shell electrons
Oxygen is in group 6, hence it has 6 outer shell electrons
Step 3: Add electrons for each hydrogen atom
Each fluorine atom contributes one electron, adding 2 electrons for 2 fluorines
Total electrons = 6 (from O) + 2 (from 2 F) = 8 electrons
Step 4: Calculate total electron pairs
Total electron pairs =28=4 electron pairs
Step 5: Deduce bonding and lone pairs
2 fluorine atoms mean 2 bonding pairs, so 4 - 2 = 2 lone pairs.
Step 6: Predict the molecular shape
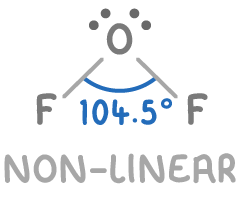
With 2 bonding and 2 lone pairs, OF2 has a bent or non-linear shape with an approximate bond angle of 104.5°.
Worked example 2 - Predicting the shape of the NH4+ ion
Predicting the shape of an ammonium (NH4+) ion involves similar steps.
Step 1: Identify the central atom
Nitrogen (N) is the central atom
Step 2: Calculate total outer shell electrons
Nitrogen is in group 5, hence it has 5 outer shell electrons
Step 3: Add electrons for each hydrogen atom
Each hydrogen atom contributes one electron, adding 4 electrons for 4 hydrogens
Total electrons = 5 (from N) + 4 (from 4 H) = 9 electrons
Step 4: Adjust for charge on the ion
The ammonium ion has a +1 charge, meaning we subtract one electron
Total electrons after charge adjustment = 9 - 1 = 8 electrons
Step 5: Calculate total electron pairs
Total electron pairs =28=4 electron pairs
Step 6: Deduce bonding and lone pairs
All 4 electron pairs are used for bonding with hydrogen atoms, leaving 0 lone pairs.
Step 7: Predict the molecular shape
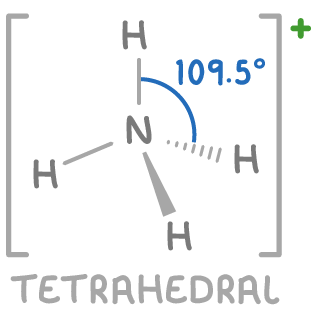
With 4 bonding pairs and 0 lone pairs, the NH4+ ion has a tetrahedral shape with bond angles of approximately 109.5°.