The Acid-base Chemistry of Transition Metal Ions
This lesson covers:
- How metal-aqua ions are formed
- Why solutions with metal-aqua ions are acidic
- Precipitation reactions of metal-aqua ions
- Amphoteric behaviour of some metal hydroxides
How metal ions become hydrated
When transitional metal compounds dissolve in water, the water molecules attach to the metal ions via coordinate covalent bonds, forming metal-aqua complex ions.
Each metal ion becomes surrounded by 6 water molecules in the aqua ion:
[M(H2O)6]n+
Where M is the metal ion and n is its charge.
These can also be written simply as Mn+(aq) where M is the metal.
For example, iron(II) forms the aqua ion [Fe(H2O)6]2+:
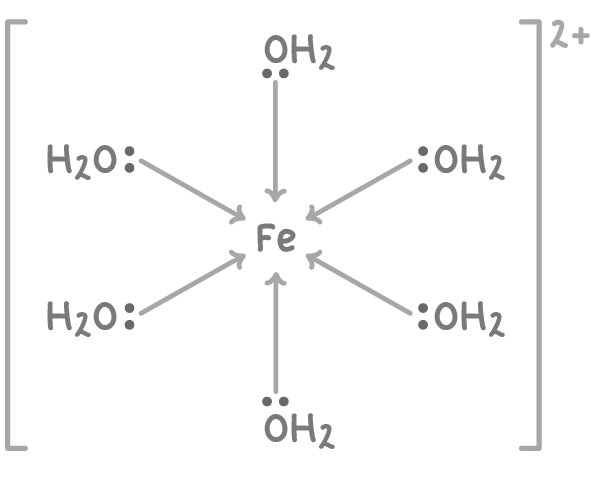
Solutions containing metal-aqua ions are acidic
Solutions containing metal-aqua ions are acidic due to their ability to undergo hydrolysis, an acid-base reaction with water. The extent of hydrolysis and the resulting acidity depend on the charge of the metal ion.
Hydrolysis of M2+(aq) ions
For example, a 2+ metal-aqua ion undergoes hydrolysis as follows:
[Fe(H2O)6]2+(aq) + H2O(l) ⇌ [Fe(H2O)5(OH)]+(aq) + H3O+(aq)
In this equilibrium, the metal-aqua ion reacts with water, releasing H3O+ ions and making the solution mildly acidic.
Hydrolysis of M3+(aq) ions
Metal-aqua ions with a 3+ charge hydrolyse to a greater extent, releasing more H3O+ ions compared to 2+ ions. For example:
[Fe(H2O)6]3+(aq) + H2O(l) ⇌ [Fe(H2O)5(OH)]2+(aq) + H3O+(aq)
The higher acidity of solutions containing M3+(aq) ions is because:
- The 3+ metal ions have a smaller ionic radius compared to 2+ metal ions, while possessing a higher charge.
- This allows it to polarise the water ligands more strongly.
- This weakens the O-H bonds of H2O so H+ ions can dissociate more easily.
Precipitation reactions
When solutions containing transition metal-aqua ions are mixed with sodium hydroxide (NaOH), aqueous ammonia (NH3), or aqueous sodium carbonate (Na2CO3), coloured precipitates are formed via ligand displacement reactions.
You need to know the formulae and colours of the precipitates formed when Cu2+, Fe2+, Fe3+, and Al3+ metal aqua ions undergo precipitation reactions with NaOH, NH3 and Na2CO3.
Precipitation using sodium hydroxide solution
With sodium hydroxide, precipitation occurs through a stepwise hydrolysis mechanism. Hydroxide ions progressively displace aqua ligands from the metal-aqua complex ion until an insoluble metal hydroxide precipitate is formed.
For example, the stepwise mechanism for the hydrolysis of M3+ aqua ions is:
- [M(H2O)6]3+(aq) + H2O(l) ⇌ [M(H2O)5(OH)]2+(aq) + H3O+(aq)
- [M(H2O)5(OH)]2+(aq) + H2O(l) ⇌ [M(H2O)4(OH)2]+(aq) + H3O+(aq)
- [M(H2O)4(OH)2]+(aq) + H2O(l) ⇌ M(H2O)3(OH)3(s) + H3O+(aq)
In each step, adding hydroxide ions shifts the position of equilibrium to the right by removing H3O+ ions. This allows progressive hydrolysis of the metal aqua complex until the insoluble hydroxide precipitates.
A similar stepwise hydrolysis mechanism occurs for M2+ metal aqua ions, just with one less step to reach the fully precipitated M(H2O)4(OH)2 hydroxide.
Precipitation using ammonia solution
When ammonia (NH3) is added to solutions containing transition metal-aqua ions, precipitation reactions also occur to form metal hydroxides.
When ammonia (NH3) dissolves in water, the following equilibrium is established:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
The hydroxide ions produced can then displace the coordinated water molecules through a stepwise hydrolysis mechanism similar to reactions with NaOH. This occurs similarly for both M2+ and M3+ metal aqua ions.
For some metal ions like Cu2+, excess ammonia causes the precipitate to dissolve again by formation of amine complex ions:
Cu(H2O)4(OH)2(s) + 4NH3(aq) ➔ [Cu(NH3)4(H2O)2]2+(aq) + 2OH-(aq) + 2H2O(l)
The formulae and colours of the hydroxide precipitates formed with Cu2+, Fe2+, Fe3+, and Al3+ metal ions are summarised below:
| Metal aqua ion | With OH-(aq) or NH3(aq) | With excess NH3(aq) |
|---|---|---|
| Cu2+ | Blue precipitate of Cu(H2O)4(OH)2 | Deep blue solution of [Cu(NH3)4(H2O)2]2+ |
| Fe2+ | Green* precipitate of Fe(H2O)4(OH)2 | No change |
| Fe3+ | Brown precipitate of Fe(H2O)3(OH)3 | No change |
| Al3+ | White precipitate of Al(H2O)3(OH)3 | No change |
*goes brown standing in air as [Fe(H2O)4(OH)2] is oxidised to [Fe(H2O)3(OH)3].
The colours of these precipitates allow the transition element ions in an unknown solution to be identified.
Precipitation using sodium carbonate solution
When sodium carbonate (Na2CO3) is added to solutions containing transition metal-aqua ions, the precipitation behavior depends on the charge of the metal ion.
Precipitation of M2+ ions
For solutions containing M2+ aqua ions, the addition of sodium carbonate results in the formation of insoluble metal carbonates. The ionic equation for this precipitation reaction is:
[M(H2O)6]2+(aq) + CO32-(aq) ➔ MCO3(s) + 6H2O(l)
Precipitation of M3+ ions
In contrast, the precipitation of carbonates does not readily occur for M3+ ions.
This is because:
- M3+ ions are stronger acids than M2+ ions so there is a higher concentration of H3O+ ions in solution.
- Instead of displacing water ligands, the carbonate ions react with the H3O+ ions according to the equation:
CO32-(aq) + 2H3O+(aq) ➔ CO2(g) + 3H2O(l)
- This removes H3O+ ions from solution, shifting the hydrolysis equilibria to the right.
[M(H2O)6]3+(aq) + 3H2O(l) ⇌ M(H2O)3(OH)3(s) + 3H3O+(aq)
- Further hydrolysis is favoured and the predominating precipitate is the hydroxide M(OH)3(H2O)3 rather than the carbonate M2(CO3)3.
The formulae and colours of the precipitates formed with Cu2+, Fe2+, Fe3+, and Al3+ metal ions are summarised below:
| Metal aqua ion | With Na2CO3(aq) |
|---|---|
| Cu2+ | Green-blue precipitate of CuCO3 |
| Fe2+ | Green precipitate of FeCO3 |
| Fe3+ | Brown precipitate of Fe(H2O)3(OH)3 / Bubbles of CO2 |
| Al3+ | White precipitate of Al(H2O)3(OH)3 / Bubbles of CO2 |
Amphoteric metal hydroxides
Some metal hydroxide precipitates formed in these reactions display amphoteric behaviour:
- They can react with acids by accepting H+ ions (acting as a Brønsted-Lowry base).
- They also react with excess OH- ions by donating H+ ions (acting as a Brønsted-Lowry acid).
Aluminium hydroxide, Al(H2O)3(OH)3 is amphoteric hydroxides because it dissolves in both acidic and basic conditions:
- In the presence of acid: Al(H2O)3(OH)3(s) + 3H+(aq) ➔ [Al(H2O)6]3+(aq)
- In the presence of base: Al(H2O)3(OH)3(s) + OH-(aq) ➔ [Al(H2O)2(OH)4]-(aq) + H2O(l)
This amphoteric solubility explains why Al(OH)3 dissolves when excess sodium hydroxide is added.
| Metal aqua ion | With OH-(aq) or NH3(aq) | With excess OH-(aq) | With excess NH3(aq) |
|---|---|---|---|
| Fe2+ | Green* precipitate of Fe(H2O)4(OH)2 | No change | No change |
| Cu2+ | Blue precipitate of Cu(H2O)4(OH)2 | No change | Deep blue solution of [Cu(H2O)2(NH3)4]2+ |
| Fe3+ | Brown precipitate of Fe(H2O)3(OH)3 | No change | No change |
| Al3+ | White precipitate of Al(H2O)3(OH)3 | Colourless solution of [Al(H2O)2(OH)4]- | No change |