The Rate-Determining Step
This lesson covers:
- What the rate-determining step is
- How the rate-determining step relates to the rate equation
- Predicting the rate equation from the rate-determining step
- Using the rate equation to deduce reaction mechanisms
- Intermediates in the rate-determining step
The slowest step determines the overall reaction rate
The rate-determining step (also known as the rate-limiting step) is the slowest step in a multi-step reaction mechanism. It dictates the overall rate of the reaction.
Just like how the flow of people exiting a crowded room is limited by how quickly they can get through the doorway, the rate of a multi-step reaction is restricted by its slowest step.
Each step in a reaction mechanism can have a different rate, but the step with the slowest rate controls how fast the reaction proceeds overall.
The rate equation reveals the rate-determining step
The rate equation provides valuable insights into the mechanism of a chemical reaction, particularly in identifying which reactants are involved in the rate-determining step.
Here are the key points to remember:
- If a reactant appears in the rate equation, it must be involved in the rate-determining step, either directly or via a substance derived from it.
- Conversely, if a reactant is absent from the rate equation, neither it nor any substance derived from it participates in the rate-determining step.
It's important to note that:
- The rate-determining step is not always the first step in the mechanism.
- The reaction mechanism usually cannot be deduced solely from the balanced equation.
Predicting the rate equation from the mechanism
The order of reaction with respect to a particular reactant indicates how many molecules of that reactant are involved in the rate-determining step.
For instance, if a reaction is second-order with respect to reactant X, then two molecules of X must be present in the rate-determining step.
Consider the following example.
The mechanism for the reaction between chlorine radicals and ozone (O3) consists of two steps:
Step 1 (slow): Cl• + O3 ➔ ClO• + O2
Step 2 (fast): ClO• + O3 ➔ Cl• + 2O2
To predict the rate equation, note that:
- Both Cl• and O3 appear in the slow, rate-determining step, so both must be in the rate equation.
- There is one Cl• radical and one O3 molecule in this step, so the reaction is first-order with respect to each.
Therefore, the rate equation takes the form: rate = k[Cl•][O3]
Using the rate equation to deduce the mechanism
Knowledge of which reactants feature in the rate-determining step can aid in deducing the reaction mechanism.
Let's examine an example.
The reaction below shows the substitution of Cl in chloromethane by the OH- nucleophile.
There are two potential mechanisms for this process.
Mechanism 1 (single-step)
A one-step process where the OH- nucleophile directly substitutes the Cl atom in a single transition state.
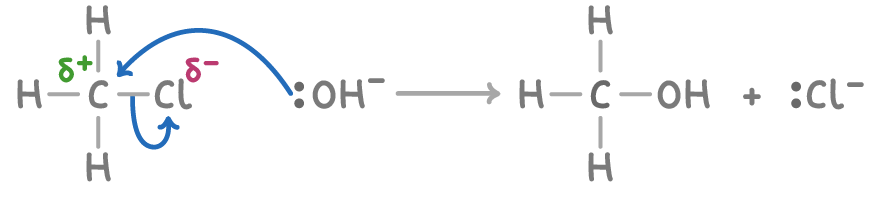
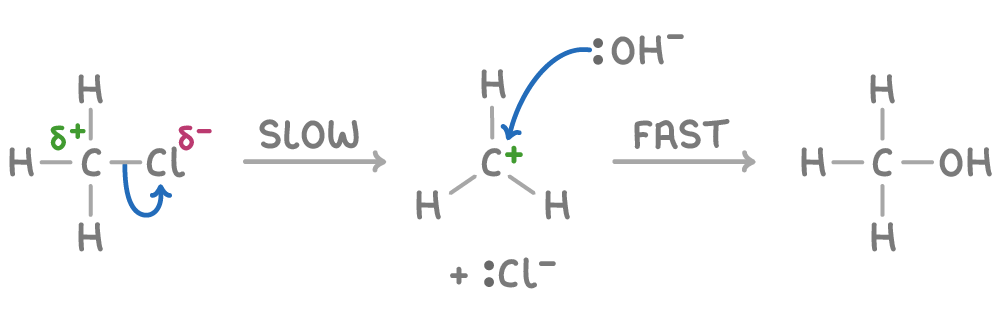
Mechanism 2 (two-step)
Step 1 (slow) - The C-Cl bond breaks, forming a carbocation intermediate and Cl-. This is likely the rate-determining step as breaking a strong C-Cl bond requires significant energy.
Step 2 (fast) - The positively charged carbocation rapidly reacts with the negatively charged OH- nucleophile to form the final product. If the OH- concentration is high, this step should occur quickly once the carbocation forms.
The experimentally determined rate equation is:
rate = k[CH3Cl]
The absence of [OH-] in this equation indicates that OH- is not involved in the rate-determining step. This supports mechanism 2 being correct, where OH- only appears in the fast second step.
Intermediates in the rate-determining step
In some cases, the rate-determining step may involve an intermediate species that is formed and consumed during a reaction, but does not appear in the overall balanced equation.
Consider this example.
The reaction 2NO(g) + O2(g) ➔ 2NO2(g) proceeds via a two-step mechanism:
Step 1: 2NO ➔ N2O2
Step 2: N2O2 + O2 ➔ 2NO2
If the rate equation is experimentally determined to be: rate = k[NO]2[O2], which step is rate-determining?
The rate equation tells us that the rate-determining step must involve:
- 2 molecules of NO
- 1 molecule of O2
Step 1 cannot be the rate-determining step as it does not involve O2. Although step 2 doesn't contain the reactants in the stoichiometry expected from the rate equation, it does involve the intermediate N2O2. This intermediate is derived from 2NO molecules, matching the rate equation.
Therefore, step 2 is the rate-determining step, even though it involves the intermediate N2O2 rather than the reactants shown in the overall equation.