Electrochemical Cells
This lesson covers:
- How batteries function as electrochemical cells
- The design and chemistry of rechargeable lithium batteries
- The components and reactions in a hydrogen-oxygen fuel cell
- The advantages and disadvantages of using fuel cells
Batteries work as electrochemical cells
Batteries are electrochemical cells that supply the electrical energy used to power various devices in our daily lives, from wristwatches to mobile phones. The key principle underpinning modern batteries is that the two electrodes have different electrode potentials. This potential difference allows the cell reaction to occur, generating electricity.
Batteries come in two main varieties:
- Non-rechargeable batteries - These are less expensive upfront but must be replaced once their stored energy is depleted. In these batteries, the cell reaction cannot be reversed.
- Rechargeable batteries - While initially more costly, these batteries can be recharged and reused multiple times, making them more economical in the long run. In rechargeable batteries, the cell reaction can be reversed by applying an external current.
Lithium batteries are rechargeable
Rechargeable batteries are commonly found in portable electronic devices such as laptops, smartphones, and even electric vehicles. Lithium-ion batteries are a popular choice due to their high energy density and rechargeability.
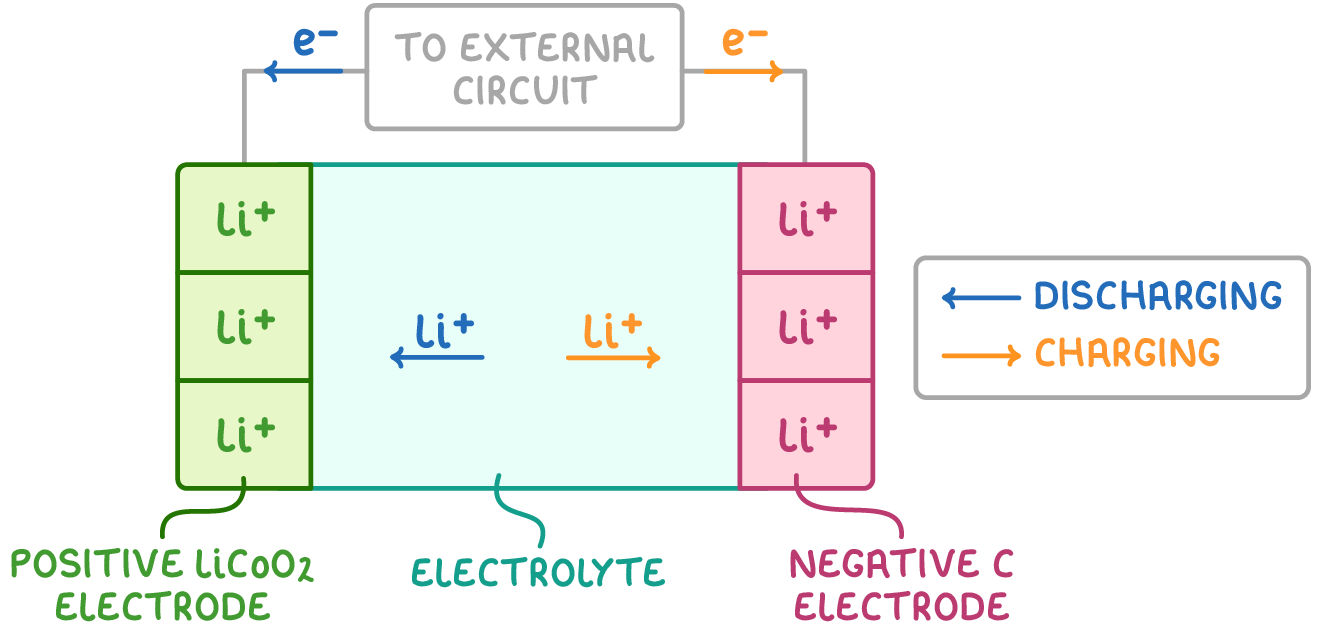
A typical lithium-ion cell consists of:
- A lithium cobalt oxide (LiCoO2) positive electrode.
- A graphite negative electrode.
- An electrolyte composed of a lithium salt dissolved in an organic solvent.
The half-equations for the electrochemical reactions in a lithium-ion battery are:
Li+ + e- ⇌ Li E⦵ = -3.04 V
CoO2 + Li+ + e- ⇌ LiCoO2 E⦵ = +0.56 V
The Li+/Li half-cell has the more negative standard reduction potential (E⦵), so it undergoes oxidation (i.e. proceeds in the reverse direction) during battery discharge.
The reactions that occur when the battery provides power are:
- At the negative electrode: Li ➔ Li+ + e-
- At the positive electrode: CoO2 + Li+ + e- ➔ Li+[CoO2]-
The overall cell potential (EMF) for this type of lithium-ion battery is calculated as:
Ecell⊖=Ereduced⊖−Eoxidised⊖
Ecell⊖=+0.56−(−3.04)=+3.60 V
To recharge these batteries, an external current is applied to drive electrons in the opposite direction around the circuit, reversing the reactions at both electrodes.
By contrast, the reactions in non-rechargeable batteries are difficult or impossible to reverse.
Fuel cells generate electricity from hydrogen and oxygen
Unlike traditional batteries, where the energy-producing chemicals are contained within the electrodes and electrolyte, fuel cells store their reactants separately and feed them into the cell as needed to produce electricity on demand. This allows fuel cells to operate continuously as long as reactants are supplied, unlike batteries which eventually run down and need to be recharged.
One such example is the alkaline hydrogen-oxygen fuel cell, a potential power source for electric vehicles.
In this type of fuel cell:
- Hydrogen and oxygen gases are supplied to separate platinum-containing electrodes
- An anion-exchange membrane separates the electrodes, allowing passage of anions (OH-) and water but not the gaseous reactants
- The electrolyte is an aqueous potassium hydroxide (KOH) solution
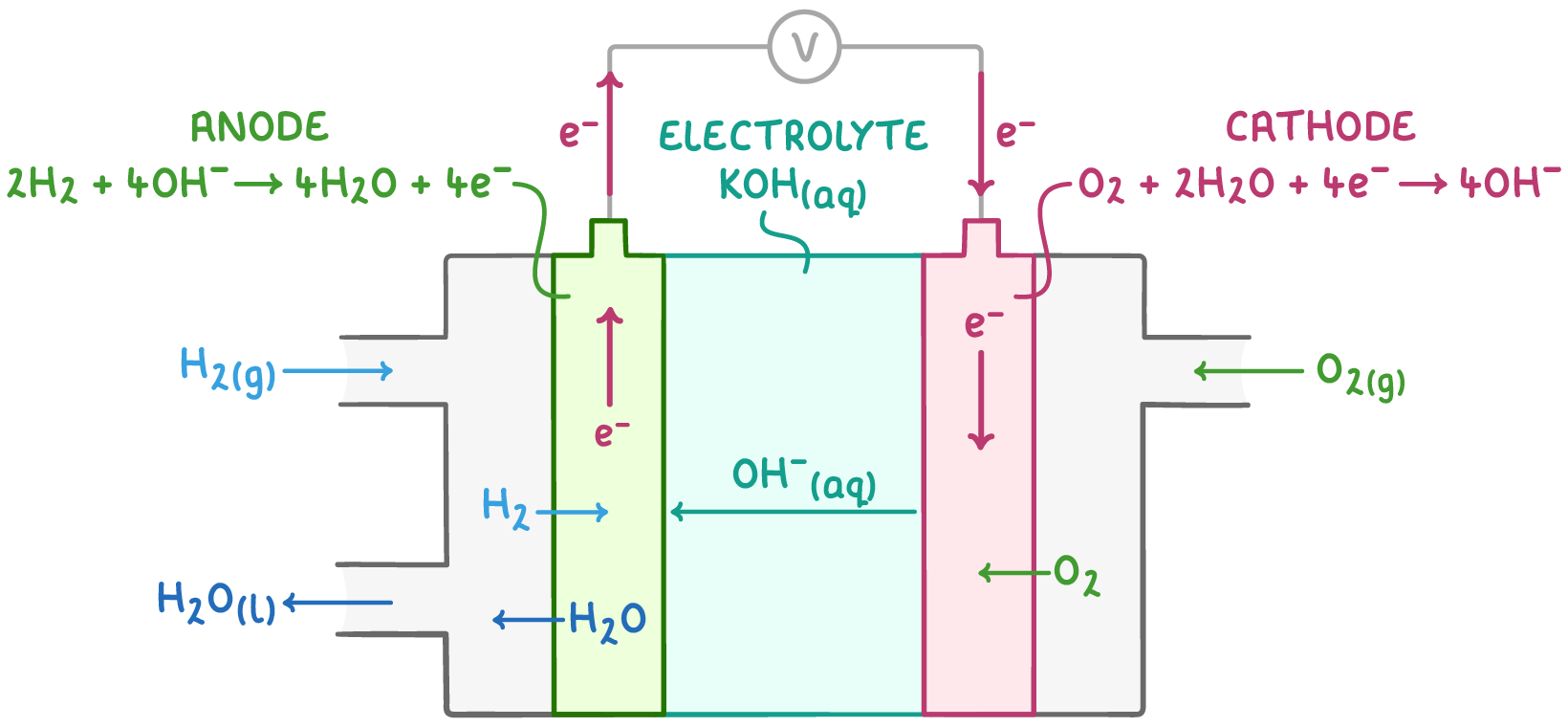
During operation, electrons flow from the negative electrode through an external circuit to the positive electrode, while OH- ions migrate through the anion-exchange membrane towards the negative electrode.
The overall reaction in the fuel cell is the formation of water from hydrogen and oxygen:
2H2(g) + O2(g) ➔ 2H2O(l)
The specific reactions at each electrode are:
- Negative electrode: 2H2(g) + 4OH-(aq) ➔ 4H2O(l) + 4e-
- Positive electrode: O2(g) + 2H2O(l) + 4e- ➔ 4OH-(aq)
Advantages and disadvantages of fuel cells
Fuel cells offer several advantages over traditional internal combustion engines in vehicles:
- Higher efficiency - Fuel cells convert a greater portion of their available energy into kinetic energy to propel the vehicle, while internal combustion engines lose a significant amount of energy as heat.
- Clean emissions - The only by-product of fuel cells is water, eliminating the release of toxic chemicals or carbon dioxide from the cell itself.
- No recharging required - As long as hydrogen and oxygen are supplied, fuel cells will continue to generate electricity without the need for recharging like batteries.
However, fuel cells also have some drawbacks:
1. Energy-intensive reactant production - Producing the required hydrogen and oxygen, typically through water electrolysis, demands significant energy input. This electricity is often generated by burning fossil fuels, so the overall process may not be carbon-neutral.
2. Flammability concerns - Hydrogen gas is highly flammable, necessitating careful handling during storage and transportation to ensure safety.