Acylation
This lesson covers:
- The structure and nomenclature of acyl chlorides
- Reactions of acyl chlorides
- The mechanism of acyl chloride reactions
- The use of acyl chlorides to manufacture aspirin
Acyl chlorides contain the functional group -COCl
Acyl chlorides, also known as acid chlorides, are a type of compound that features the functional group -COCl. These compounds are derived from carboxylic acids, where the hydroxyl (-OH) group is replaced by a chlorine atom (-Cl).
Similar to aacyl chlorides, acid anhydrides and amides are also derivatives of carboxylic acids.

To name an acyl chloride, the suffix "-oic acid" of the parent carboxylic acid is replaced with "-oyl chloride". For example:
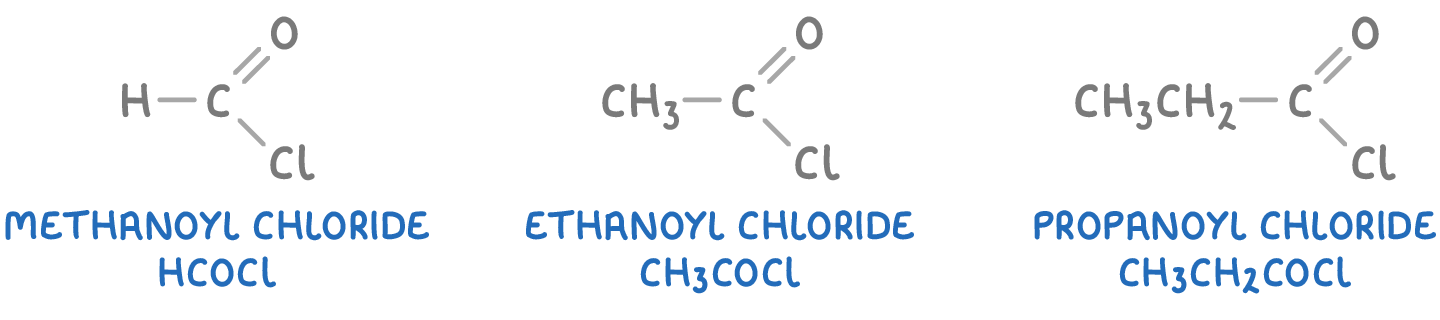
When numbering the carbon chain in an acyl chloride, the numbering starts from the end closest to the -COCl group, similar to carboxylic acids.
Reactions of acyl chlorides
Acyl chlorides are highly reactive due to their polar C=O bond and the chlorine atom, which is readily displaced.
They can react with:
- Water - This reaction is vigorous, even at low temperatures, and reforms the carboxylic acid. The reaction also produces steamy fumes of hydrogen chloride gas. For example, ethanoyl chloride is hydrolysed to ethanoic acid according to the equation:
CH3COCl + H2O ➔ CH3COOH + HCl
- Alcohols - At room temperature, this reaction is vigorous, producing an ester and HCl. For example, ethanoyl chloride reacts with ethanol to form ethyl ethanoate according to the equation:
CH3COCl + C2H5OH ➔ CH3COOC2H5 + HCl
- Ammonia - This reaction occurs violently at room temperature, resulting in a primary amide and NH4Cl. For example, ethanoyl chloride reacts with ammonia to form ethanamide according to the equation:
CH3COCl + 2NH3 ➔ CH3CONH2 + NH4Cl
- Primary amines - This reaction occurs violently at room temperature, resulting in a secondary amide and (CH3)2NH2+Cl-. For example, ethanoyl chloride reacts with methylamine to form N-methylethanamide according to the equation:
CH3COCl + 2CH3NH2 ➔ CH3CONHC2H5 + (CH3)2NH2Cl
Acyl chlorides react via addition-elimination
The reaction mechanism for acyl chlorides involves nucleophilic addition-elimination:
- Addition - A nucleophile attacks the carbonyl carbon, which carries a partial positive charge, leading to the formation of a tetrahedral intermediate.
- Elimination - The chloride ion leaves, and the carbonyl group is reformed, with HCl being released.
For example, the mechanism using water as the nucleophile is shown below (R = alkyl group):
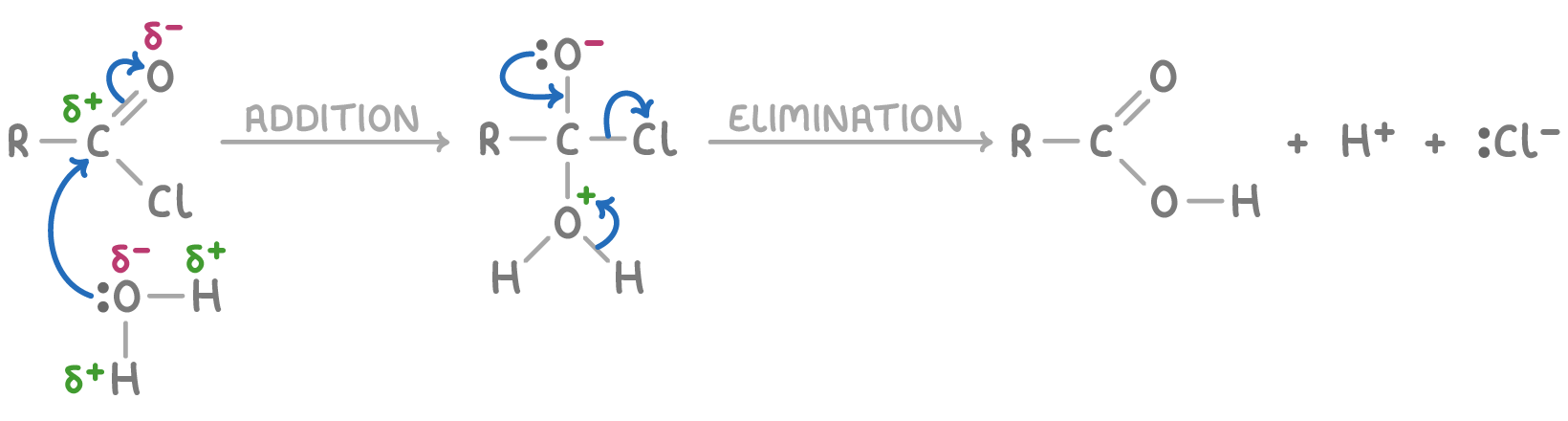
Manufacturing aspirin
Aspirin, or acetylsalicylic acid, is produced industrially by reacting salicylic acid with an excess of ethanoic anhydride, a derivative of acyl chloride.
This method is preferred over using ethanoyl chloride for several reasons:
- Ethanoic anhydride is cheaper than ethanoyl chloride.
- It is less corrosive, reducing the need for specialised equipment.
- It reacts more slowly with water, allowing for better control during the manufacturing process.
- It produces ethanoic acid as a by-product, which is less hazardous than the corrosive hydrogen chloride gas generated by ethanoyl chloride.