Ionisation Energies
This lesson covers:
- What ionisation energy is
- The factors that affect ionisation energy
- Trends in first ionisation energy down groups and across periods
- Successive ionisation energies
Ionisation involves removing electrons
Ionisation refers to the process of removing one or more electrons from an atom or molecule. This requires an input of energy, so ionisation is an endothermic process.
The first ionisation energy is the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions.
For example, the equation that represents the first ionisation energy (IE1) of magnesium is:
Mg(g) ➔ Mg+(g) + e− ΔHIE1 = +738 kJ mol−1
Since ionisation requires energy input, the values for ionisation energies are always positive.
Factors affecting ionisation energy
The ionisation energy of an atom depends on how strongly its outermost electrons are attracted to the nucleus. There are three key factors that affect this electrostatic attraction:
- Nuclear charge - Atoms with more protons in their nucleus have a stronger positive charge. This creates stronger electrostatic attraction between the nucleus and the outer electrons.
- Atomic radius - Electrostatic attraction drop off steeply with increasing distance. Electrons in smaller atoms are held closer to the nucleus so the attraction is greater.
- Electron shielding - Inner electron shells shield the outermost electrons from the full attractive force of the nucleus, reducing the effective nuclear charge experienced by the outer electrons. More electron shells provide more shielding.
In summary, low shielding and small atomic size lead to high ionisation energies, as the outermost electrons experience strong electrostatic attraction from the nucleus. Removing these tightly-held electrons requires substantial energy input.
Trends in first ionisation energy
Ionisation energies show clear trends down groups and across periods of the periodic table due to the combined effects of nuclear charge, atomic radius, and electron shielding.
Down groups:
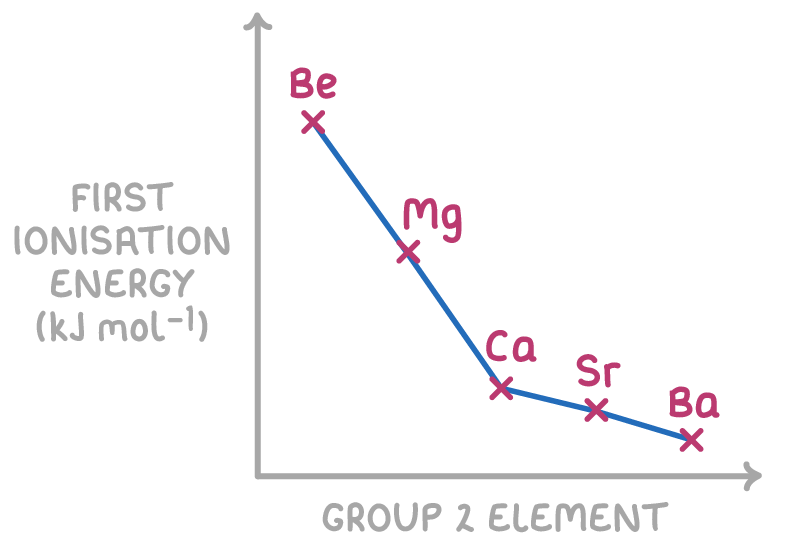
Ionisation energy decreases down groups because:
- Nuclear charge - Increases down the group as more protons are added, increasing attraction for electrons
- Atomic radius - Increases down the group as more electron shells are added, moving electrons away from nucleus
- Electron shielding - Increases down group as more inner electron shells reduce nuclear attraction
The atomic radius and shielding effects down groups are greater than the nuclear charge effect, leading to an overall decrease in ionisation energies as you move down a group.
Across periods:
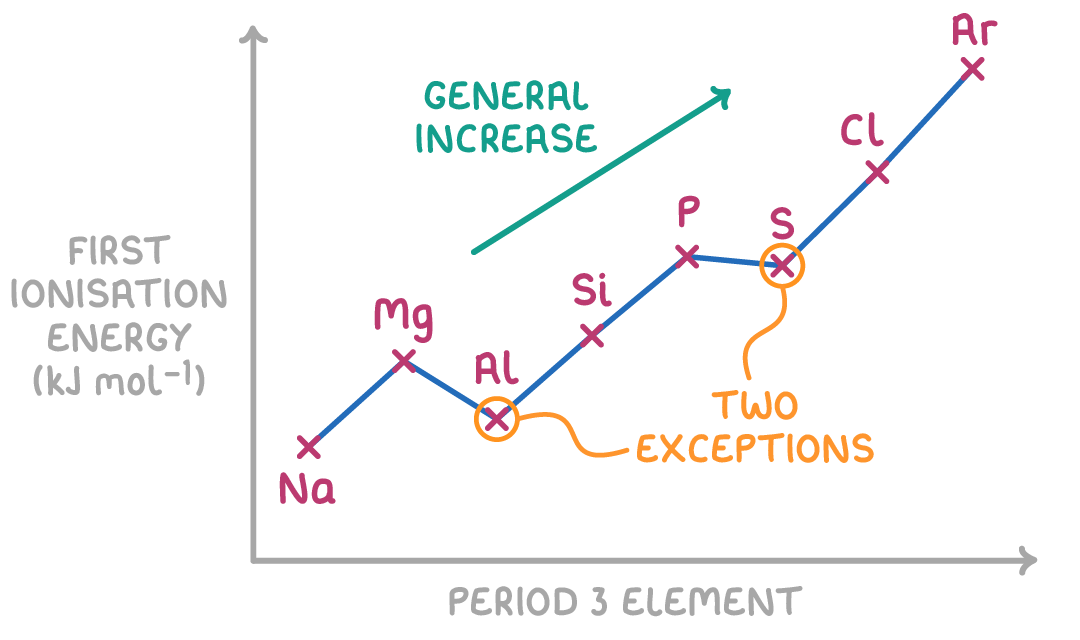
Ionisation energy generally increases across periods because:
- Nuclear charge - Increases as more protons added across periods
- Atomic radius - Decreases across periods as extra electrons added to same shell
- Electron shielding - Stays similar across periods with no extra inner shells
The increasing nuclear charge effect outweighs the similar shielding across periods, so ionisation energies generally increase as you move across a period.
There are two exceptions between groups 2-3 and groups 5-6. These drops occur due to electron configuration effects.
The drop between groups 2 and 3
This drop occurs because:
- In group 3, the electron is removed from a p orbital rather than an s orbital like in group 2.
- p orbitals have slightly higher energy than s orbitals, so the outermost electron is on average further from the nucleus.
- The p orbital also experiences additional shielding from the nucleus provided by the s electrons.
- As a result, less energy is required to remove the outermost p electron from the group 3 element compared to removing the outermost s electron from the group 2 element.
For example, aluminium has a lower first ionisation energy than magnesium.
| Element | Group | Electronic configuration | First ionisation energy (kJ mol-1) |
|---|---|---|---|
| Magnesium | 2 | [Ne] 3s2 | 738 |
| Aluminium | 3 | [Ne] 3s2 3p1 | 578 |
The drop between groups 5 and 6
This drop occurs because:
- In group 5, the electron is removed from a singly occupied orbital.
- In group 6, the electron is removed from an orbital containing two electrons.
- The paired electrons in the group 6 element experience greater electron-electron repulsion.
- As a result, less energy is needed to remove one of these paired electrons in the group 6 element compared to the unpaired electron in the group 5 element.
For example, sulfur has a lower first ionisation energy than phosphorus.
| Element | Group | Electronic configuration | First ionisation energy (kJ mol-1) |
|---|---|---|---|
| Phosphorus | 5 | [Ne] 3s2 3p3 | 1,011 |
| Sulfur | 6 | [Ne] 3s2 3p4 | 999 |
Successive ionisation energies
Electrons can be sequentially removed until only the bare nucleus remains. The energy to remove each successive electron is called the successive ionisation energies.
For example, the second ionisation energy is the energy needed to remove 1 electron from each ion in 1 mole of gaseous 1+ ions to form 1 mole of gaseous 2+ ions.
For example, the equation that represents the second ionisation energy (IE2) of magnesium is:
Mg+(g) → Mg2+(g) + e− ΔHIE2 = +1,451 kJ mol−1
Successive ionisation energies provide evidence for electron shell structure:
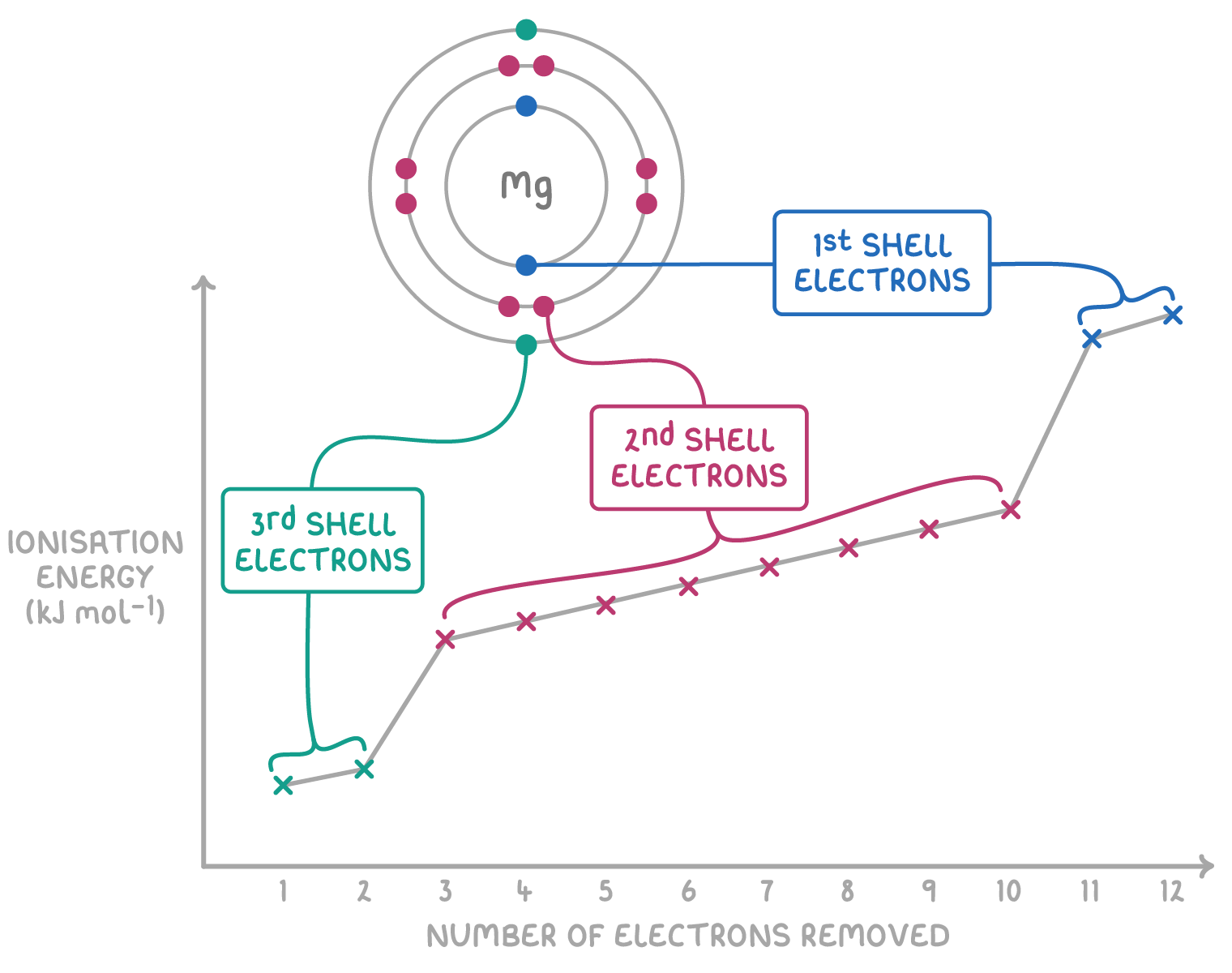
Successive ionisation energies increase within the same shell:
- As successive electrons are removed from the same shell, the remaining electrons experience greater electrostatic attraction to the increasingly positive nucleus. This increased nuclear attraction requires more energy to remove the next electron from that shell.
- For magnesium, the second ionisation energy (1,450 kJ mol-1) is slightly higher than the first (740 kJ mol-1) as these electrons are both being removed from the 3s subshell.
There are large jumps in successive ionisation energy between shells:
- When reaching a new inner electron shell, there is a big increase in the ionisation energy needed to remove the first electron in that new shell. This happens because the attraction to the nucleus is much greater for inner shell electrons closer to the nucleus.
- For magnesium, there is a large jump from the second ionisation energy (1,450 kJ mol-1) to the third (7,730 kJ mol-1) as the 3s subshell is now full, requiring the next electron to be removed from the inner 2p subshell.