Isotopes and Relative Atomic Mass
This lesson covers:
- What elements and isotopes are
- Comparing chemical and physical properties of isotopes
- Relative masses of atoms and molecules
- Calculating relative atomic mass from isotopic abundances
Isotopes have different numbers of neutrons
Elements are defined as:
Substance made up of only one type of atom, where all the atoms of a substance have the same number of protons.
Isotopes are defined as:
Atoms of the same element that have the same number of protons but different numbers of neutrons.
Most elements are made up of a mixture of isotopes.
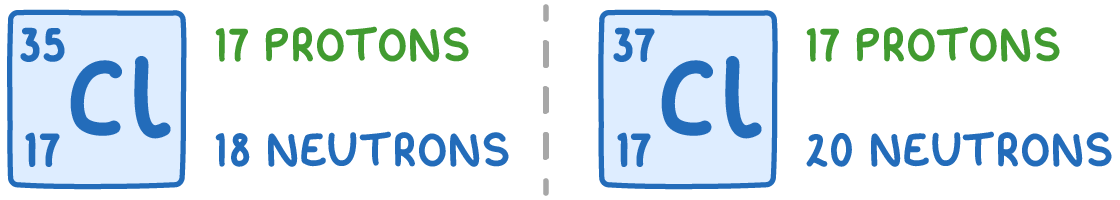
For example, chlorine has two main naturally occurring isotopes: 35Cl and 37Cl:
Both isotopes have the same number of protons (17) and electrons (17) but different mass numbers due to the different numbers of neutrons.
Chemical vs physical properties of isotopes
Chemical properties:
- Isotopes have the same electron configuration.
- This means they have the same chemical properties (e.g. reactivity).
Physical properties:
- Isotopes have slightly different physical properties (e.g. mass and density).
- This is because physical properties depend on atomic mass.
Relative masses compare to carbon-12
Atoms have tiny absolute masses that are impractical to measure directly. Instead, the mass of one atom is compared to 1/12th the mass of a carbon-12 atom. This is the atom's relative mass.
Key definitions:
Relative isotopic mass - The mass of an atom of an isotope compared to 1/12 the mass of a carbon-12 atom.
- Relative isotopic mass is specific to a particular isotope of an element.
- It is usually very close to being a whole number.
- At A level, relative isotopic masses are rounded to one decimal place.
- E.g. The relative isotopic masses of the two main chlorine isotopes (35Cl and 37Cl) are 35.0 and 37.0 respectively.
Relative atomic mass (Ar) - The weighted mean mass of an atom of an element compared to 1/12 the mass of a carbon-12 atom.
- Ar is an average of the masses of all the isotopes of an element, weighted according to their natural abundances.
- Since it's an average, Ar is not usually a whole number.
- The next section explains how to calculate relative atomic mass from isotopic abundances.
Relative molecular mass (Mr) - The average mass of a molecule compared to 1/12 the mass of a carbon-12 atom.
- For simple covalent molecules, Mr is calculated by adding up the Ar values of all the atoms in one molecule.
- E.g. For ethanol (C2H5OH): Mr(C2H5OH) = (2 × Ar of C) + (6 × Ar of H) + (1 × Ar of O) = (2 × 12.0) + (6 × 1.0) + (1 × 16.0) = 24.0 + 6.0 + 16.0 = 46.0
Relative formula mass - Used for ionic compounds. It is calculated by adding up the Ar values of all the ions in one formula unit.
- E.g. For calcium fluoride (CaF2): Mr(CaF2) = (1 × Ar of Ca) + (2 × Ar of F) = (1 × 40.1) + (2 × 19.0) = 40.1 + 38.0 = 78.1
Calculating Ar from isotopic abundances
To calculate the relative atomic mass (Ar) from isotopic abundances, use this formula:
Ar= sum of abundance of all isotopes sum of (isotopic mass × isotopic abundance)
Worked example: 1 - Calculating the relative atomic mass of chlorine from its isotopic abundances
Chlorine has two main isotopes, 35Cl with an abundance of 75% and 37Cl with an abundance of 25%.
Calculate the relative atomic mass (Ar) of chlorine.
Step 1: For each isotope, multiply its relative mass by its percentage abundance.
- For 35Cl: 35.0 × 75 = 2,625
- For 37Cl: 37.0 × 25 = 925
Step 2: Add up all the values from step 1 to get the numerator of the fraction.
2,625 + 925 = 3,550
Step 3: Add up the abundances of all isotopes to get the denominator of the fraction. If abundances are percentages, this will be 100.
75 + 25 = 100
Step 4: Divide numerator by denominator to get Ar.
Ar =1003,550=35.5
Therefore, the relative atomic mass of chlorine is 35.5.
Worked example: 2 - Calculating the relative atomic mass of magnesium from its isotopic abundances
Magnesium has three main isotopes: 24Mg with an abundance of 79%, 25Mg with an abundance of 10%, and 26Mg with an abundance of 11%.
Calculate the relative atomic mass (Ar) of magnesium.
Step 1: For each isotope, multiply its relative mass by its percentage abundance.
- For 24Mg: 24.0 × 79 = 1,896
- For 25Mg: 25.0 × 10 = 250
- For 26Mg: 26.0 × 11 = 286
Step 2: Add up all the values from step 1 to get the numerator of the fraction.
1,896 + 250 + 286 = 2,432
Step 3: Add up the abundances of all isotopes to get the denominator of the fraction. If abundances are percentages, this will be 100.
79 + 10 + 11 = 100
Step 4: Divide numerator by denominator to get Ar.
Ar =1002,432=24.32
Therefore, the relative atomic mass of magnesium is 24.3.