Electronegativity - Bond Polarity in Covalent Bonds
This lesson covers:
- What electronegativity is
- The factors affecting electronegativity
- Trends in electronegativity
- Polar bonds, polar molecules, and dipoles
- How electronegativity differences predict bond type
Electronegativity - the ability of atoms to attract bonding electrons
Electronegativity is a measure of an atom's ability to attract the shared electron pair in a covalent bond towards itself.
- Fluorine is the most electronegative element, followed by oxygen, nitrogen and chlorine.
- Electronegativity is measured on the Pauling scale. A higher value on this scale means an atom is more electronegative.
| Element | H | C | N | O | F | Cl |
|---|---|---|---|---|---|---|
| Electronegativity (Pauling scale) | 2.1 | 2.5 | 3.0 | 3.5 | 4.0 | 3.0 |
Factors affecting electronegativity
The electronegativity of an atom is influenced by:
- Atomic radius - Smaller atoms are more electronegative because their electrons are closer to the nucleus, resulting in a stronger electrostatic attraction.
- Nuclear charge - A higher positive charge in the nucleus increases the strength of the electrostatic attraction between the nucleus and the electrons, making an atom more electronegative.
- Shielding - Electrons in inner shells can weaken the electrostatic attraction between the nucleus and the outer shell electrons, reducing an atom's electronegativity.
Trends in electronegativity across the periodic table
Electronegativity patterns are clear across periods and down groups in the periodic table.
Electronegativity increases across a period
- This trend occurs because the atomic radius decreases while the nuclear charge increases, resulting in a stronger electrostatic attraction between the nucleus and the electrons in the outer shell.
- The effect of an increasing nuclear charge dominates over the decreasing atomic radius, leading to an increase in electronegativity.
Electronegativity decreases down a group
- Down a group, although the nuclear charge increases, the atomic radius increases more significantly, and the number of inner shell electrons shielding the outer electrons also increases.
- The increased distance between the nucleus and the outer electrons, combined with greater shielding, weakens the electrostatic attraction between them. As a result, electronegativity decreases despite the increase in nuclear charge.
Polar bonds arise from electronegativity differences
Differences in electronegativity between bonded atoms can cause polar bonds:
- In a covalent bond between atoms with different electronegativities, the bonding electrons are more strongly attracted to the more electronegative atom. This unequal sharing of electrons makes the bond polar.
- Polar bonds have a permanent electric dipole. A dipole is a separation of positive and negative charges within a polar covalent bond or polar molecule, resulting from an uneven distribution of electrons.
- In a polar bond, the more electronegative atom aquires a slight negative charge (δ-) and the less electronegative atom acquires a slight positive charge (δ+). The bigger the difference in electronegativity, the more polar the bond.
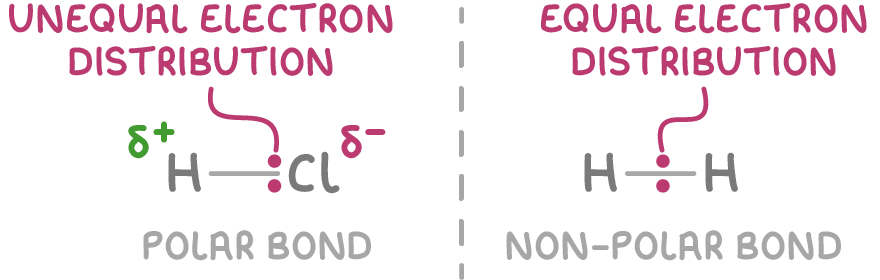
Bonds between atoms of the same element, like H2 and Cl2, are non-polar because the electrons are shared equally.
Bonds between atoms with similar electronegativity, such as carbon and hydrogen, are essentially non-polar due to an even electron distribution.
Charge is unevenly distributed in polar molecules
The overall polarity of a molecule depends on how its polar bonds are arranged in 3D:
- Molecules like CCl4 are non-polar because their symmetric arrangement causes their dipoles to cancel out, even though they have polar bonds. In CCl4, the four polar C-Cl bonds are arranged tetrahedrally, resulting in the dipoles cancelling each other out and no net dipole for the molecule.
- Molecules like CHCl3 are polar because their asymmetric arrangement doesn't allow their dipoles to cancel out, leading to a net dipole. In CHCl3, the three polar C-Cl bonds and one C-H bond are arranged tetrahedrally, but the difference in electronegativity between H and Cl causes the dipoles to not fully cancel each other, resulting in a net dipole for the molecule.
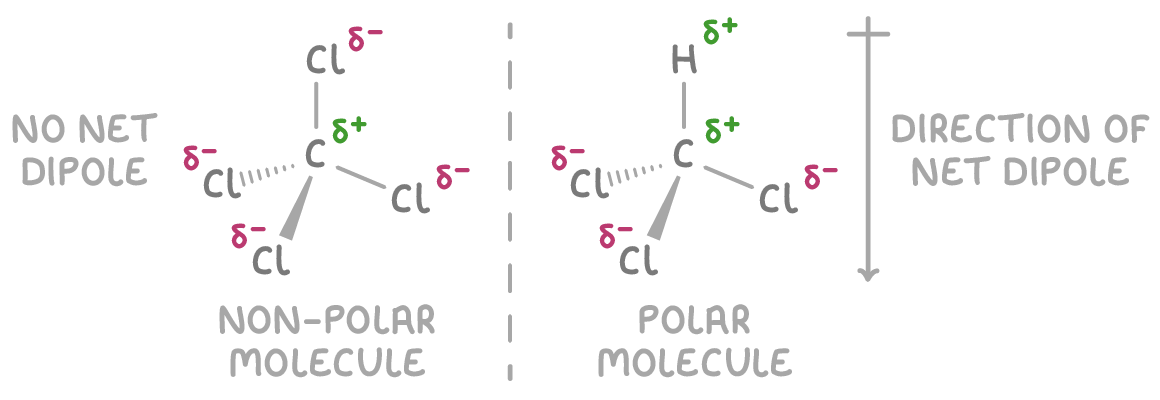
To determine if a molecule has an overall dipole, visualise its 3D structure and asses if the individual bond dipoles cancel each other out.
Electronegativity differences predict bond type
The type of bond formed between two atoms can be predicted by the difference in their electronegativities:
- When the electronegativity difference is zero, as in diatomic molecules like H2 and O2 where the atoms are identical, the bonding electrons are shared equally, resulting in a pure (non-polar) covalent bond.
- As the electronegativity difference increases, the bond becomes more polar covalent. For example, in HCl, the chlorine atom attracts the bonding electrons more strongly than the hydrogen atom, leading to a polar covalent bond.
- When the electronegativity difference is very large, the bond is considered ionic. In this case, the more electronegative atom effectively takes the bonding electrons from the less electronegative atom. An example is NaCl, where the chlorine atom takes an electron from the sodium atom, forming Na⁺ and Cl⁻ ions.
Most compounds fall somewhere between these extremes, exhibiting a mix of ionic and covalent character depending on the electronegativity difference between the bonded atoms.
| Difference in electronegativity | Bond type | Example |
|---|---|---|
| Less than 1.0 | Non-polar covalent | H₂ |
| 1.0 to 2.0 | Polar covalent | HCl |
| Greater than 2.0 | Ionic | NaCl |