Introduction to Amino Acids
This lesson covers:
- The structure of amino acids
- Naming of amino acids
- Zwitterions
Amino acids contain amine and acid groups
Amino acids consist of both amine and acid functional groups:
- An amino group (-NH2)
- A carboxylic acid group (-COOH)
These groups are attached to the same carbon atom, the α-carbon, in α-amino acids.
The general structure of an α-amino acid is illustrated as:
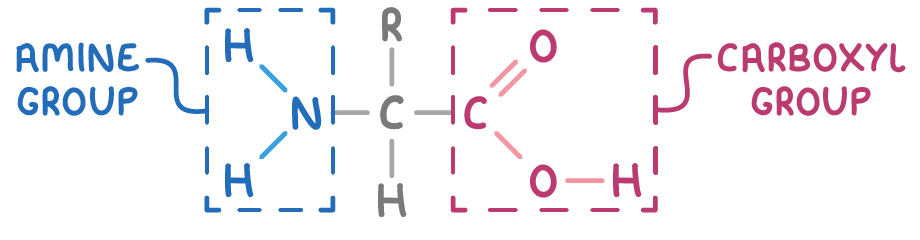
Here, R denotes a variable organic substituent i.e. an alkyl or aryl group, which is known as the side chain.
Naming amino acids
Amino acids are named using two methods:
- Common names, for example, glycine and alanine
- Systematic IUPAC names, which are based on the carbon backbone
The steps to name an amino acid systematically are:
- Identify the longest carbon chain that includes the carboxyl carbon.
- Start numbering the carbons from the carboxyl carbon, assigning it the number 1.
- Specify the position and identity of the amine substituent.
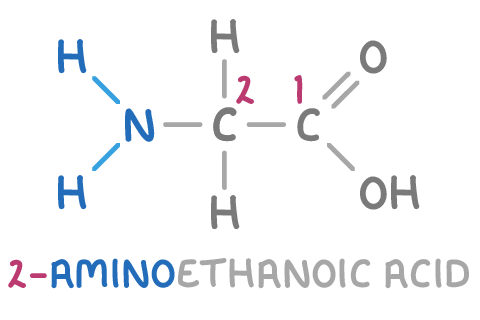
For example, the systematic name of the amino acid glycine (shown above) is 2-aminoethanoic acid, indicating it has two carbons, with NH2 attached to carbon 2.
Amino acids as zwitterions
Amino acids can form zwitterions - molecules that contain both positive and negative charges within the same structure.
Specifically, a zwitterion forms via:
- Amine group protonation - The NH2 group gains a proton to become positively charged NH3+.
- Carboxyl group deprotonation - The COOH group loses a proton to become negatively charged COO-.
These opposite charges balance out, giving an overall neutral charge. This process involves an internal transfer of a proton (H+) from the carboxyl group to the amine group.
Whether the zwitterion forms depends on the pH:
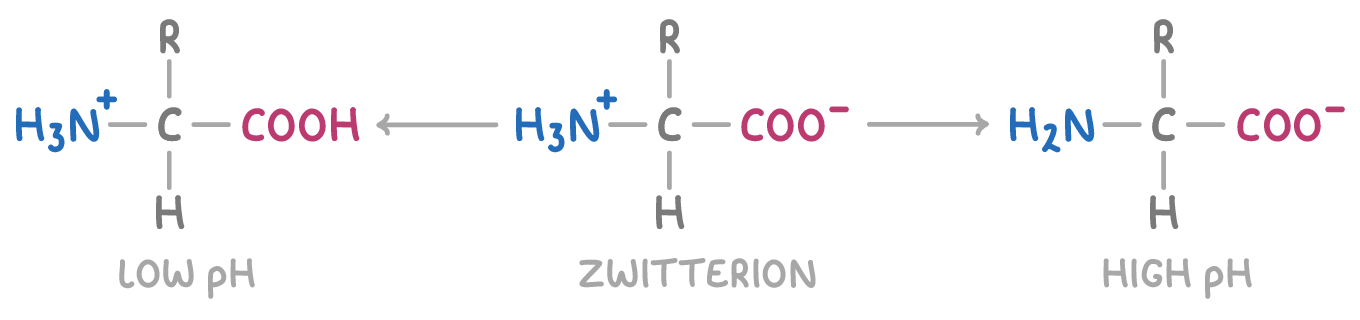
- Lower pH (acidic) - The COOH group gets deprotonated
- Neutral pH - The zwitterion itself forms as both the carboxyl and amino groups ionise
- Higher pH (alkaline) - The NH3+ group loses its proton
The existence of these pH-dependent zwitterions demonstrates that amino acids are amphoteric. They can act as both acids (via the carboxyl group) and bases (via the amine group).
The exact pH where the zwitterion forms depends on the amino acid's R-group. Different R-groups alter the relative acidity and basicity of the functional groups.