Reactions of the Carbonyl Group in Aldehydes & Ketones
This lesson covers:
- Oxidation of aldehydes to carboxylic acids
- Reduction of aldehydes and ketones to alcohols
- Hydroxynitrile formation
Reactions of the Carbonyl Group in Aldehydes & Ketones
This lesson covers:
- Oxidation of aldehydes to carboxylic acids
- Reduction of aldehydes and ketones to alcohols
- Hydroxynitrile formation
- Why some reactions produce racemic mixtures
Aldehydes are easily oxidised to carboxylic acids
Aldehydes can be oxidised by oxidising agents such as acidified potassium dichromate(VI) to form carboxylic acids. The colour change observed during this oxidation is from orange to green, as the Cr(VI) in the dichromate ion is reduced to Cr(III). However, ketones do not undergo oxidation under the same conditions, so colour change is seen.
For example, methanal is oxidised to methanoic acid:
HCHO + [O] ➔ HCOOH
By contrast, propanone does not react with oxidising agents:
CH3COCH3 + [O] ➔ no reaction
This difference in reactivity occurs because the hydrogen atom attached to the carbonyl carbon in an aldehyde is weakly acidic, so it is easily displaced. By contrast, ketones lack that weakly acidic hydrogen, so oxidation does not occur.
Reduction of aldehydes and ketones to alcohols
Aldehydes and ketones can be reduced to primary and secondary alcohols respectively using reducing agents like sodium borohydride (NaBH4) dissolved in a mixture of water and methanol.
Carbonyl compounds undergo nucleophilic addition reactions because the carbonyl carbon is electrophilic due to the polarisation of the C=O double bond, which makes it susceptible to attack by nucleophiles.
The reduction reactions are:
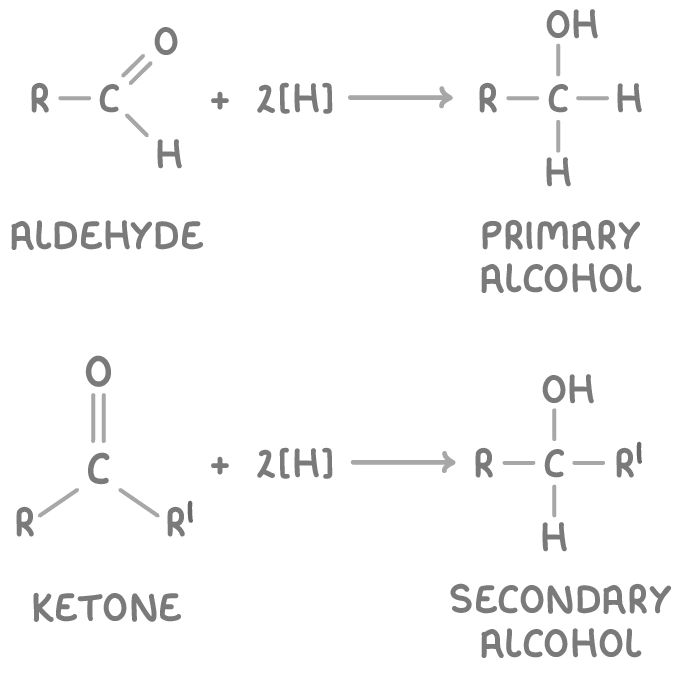
Where:
- [H] represents the reducing agent.
- R and R’ represent alkyl groups.
Mechanism of carbonyl reduction
The reduction of carbonyl compounds, such as ethanal, to alcohols follows a nucleophilic addition mechanism:
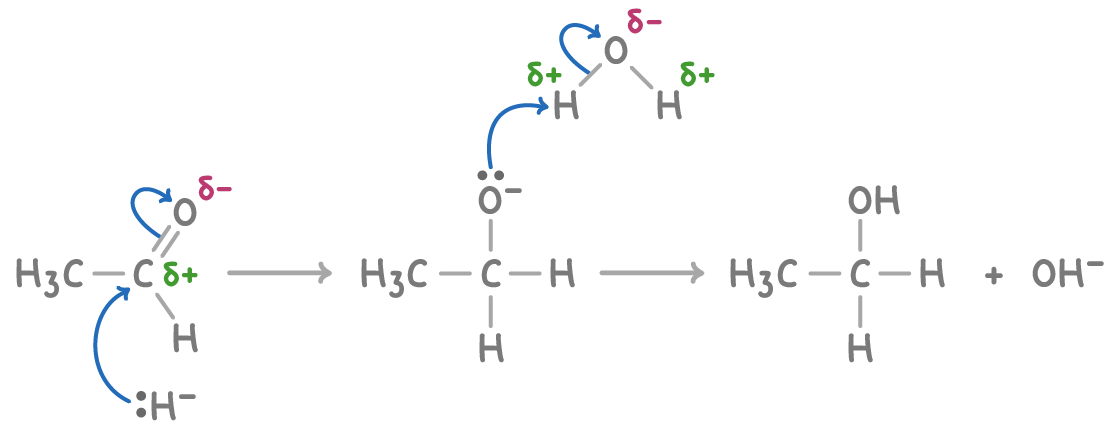
- The reducing agent provides hydride ions (H-) which act as nucleophiles.
- The hydride ion attacks the partially positive carbonyl carbon, forming a new C-H bond.
- Protons from the solvent, such as water, are added to the oxygen, forming the hydroxyl group (-OH).
Aldehydes and ketones form hydroxynitriles
Aldehydes and ketones can react with hydrogen cyanide (HCN) in nucleophilic addition reactions to produce hydroxynitriles which conains both the cyano (-CN) and hydroxy (-OH) substituents. This reaction is useful because it increases the length of the carbon chain by 1 carbon atom.
The reactions are:
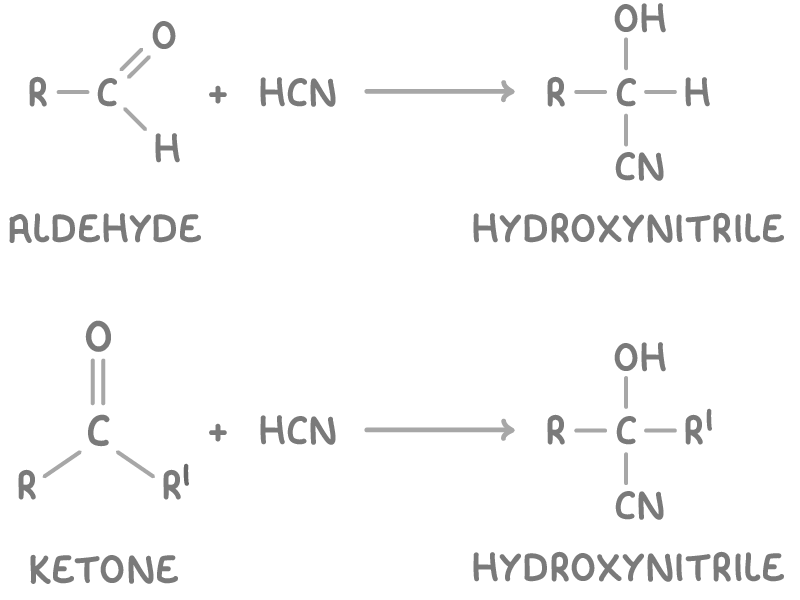
Where
- R and R’ represent alkyl groups.
Hydrogen cyanide is toxic so it is often generated in situ by mixing sodium cyanide or potassium cyanide with sulfuric acid.
Mechanism of hydroxynitrile formation
The formation of hydroxynitriles from carbonyl compounds, such as ethanal, follows a nucleophilic addition mechanism:
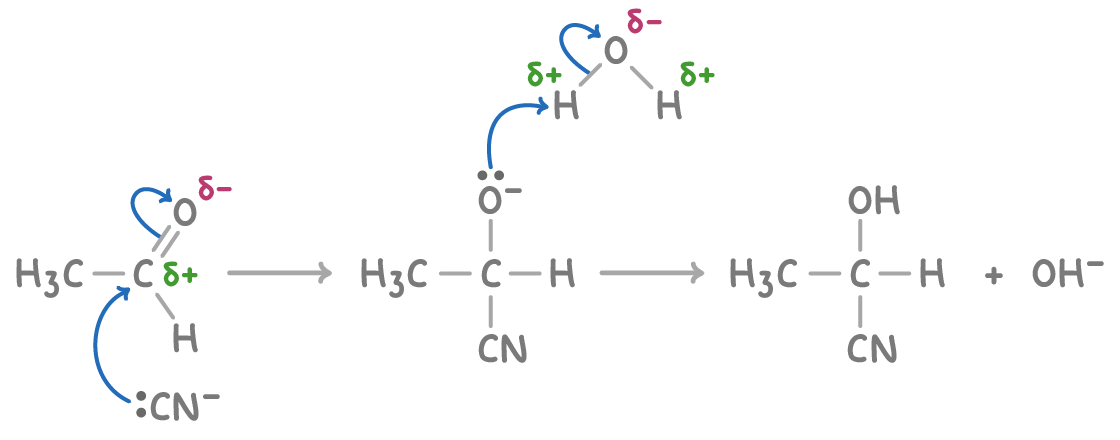
- CN- attacks the partially positive carbonyl carbon, transferring the electrons to oxygen.
- Protonation of oxygen by water (or acid) to give the -OH group.
Aldehydes and unsymmetrical ketones form racemic mixtures
Aldehydes and unsymmetrical ketones contain a planar carbonyl group (C=O) which allows attacking reagents to approach from either side with equal likelihood. This leads to the formation of racemic mixtures containing both enantiomers in equal amounts.
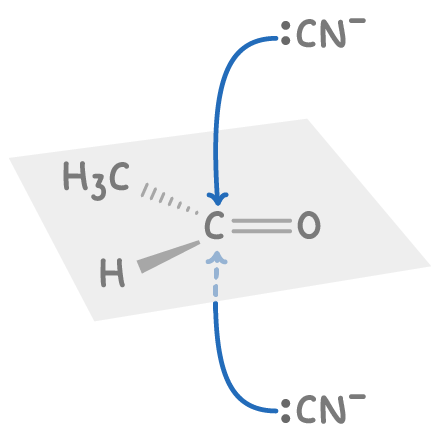
For example, when ethanal reacts with acidified potassium cyanide:
- The planar C=O double bond allows the CN- nucleophile to attack from either side of the plane.
- Attacking from above or below the plane produces two different enantiomers.
- Since both directions of attack are equally likely, the reaction yields equal amounts of both enantiomers of 2-hydroxypropanenitrile, resulting in a racemic mixture.
In contrast, symmetrical ketones do not form racemic mixtures when attacked by nucleophiles. Due to their symmetry, attack from either side of the carbonyl group produces the same product rather than two different enantiomers. For instance, when propanone reacts with acidified potassium cyanide, the product is the non-chiral molecule 2-hydroxy-2-methylpropanenitrile.