Introduction to Aldehydes & Ketones
This lesson covers:
- The carbonyl group in aldehydes and ketones
- Synthesising aldehydes and carboxylic acids from primary alcohols
- Synthesising ketones from secondary alcohols
Aldehydes and ketones contain a carbonyl group
Aldehydes and ketones are both carbonyl compounds, meaning they contain a carbon-oxygen double bond called the carbonyl group.
The key difference lies in the position of this functional group:
- Aldehydes have their carbonyl group at the end of a carbon chain. Their names end in -al.
- Some common examples of aldehydes are:
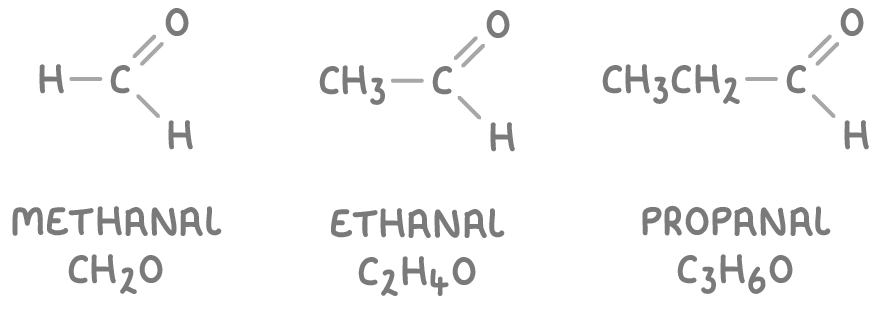
- Ketones have their carbonyl group within the carbon chain. Their names end in -one and often have a number indicating the carbon with the C=O group.
- Some common examples of ketones are:
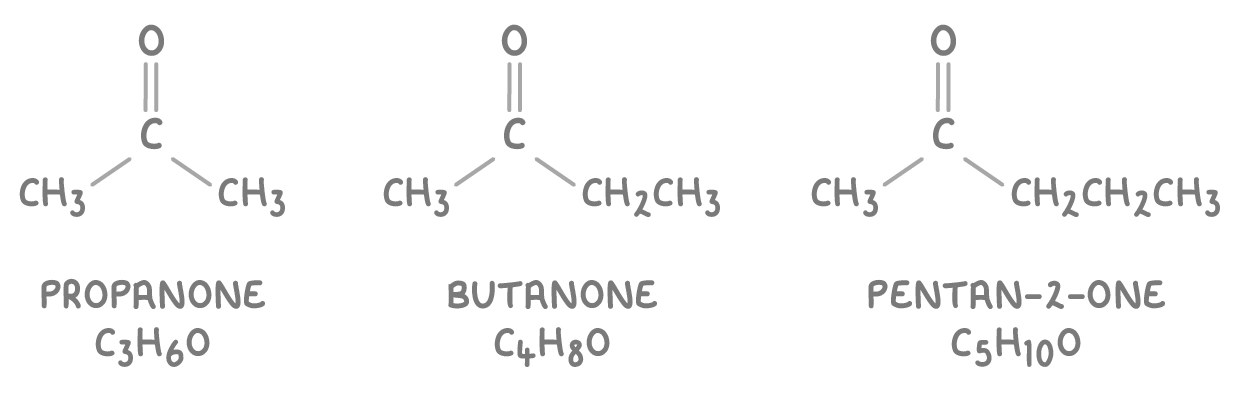
Oxidising primary alcohols to aldehydes and carboxylic acids
Primary alcohols can be oxidised to aldehydes and further to carboxylic acids using acidified potassium dichromate(VI) (K2Cr2O7) as an oxidising agent.
The oxidation reactions are:
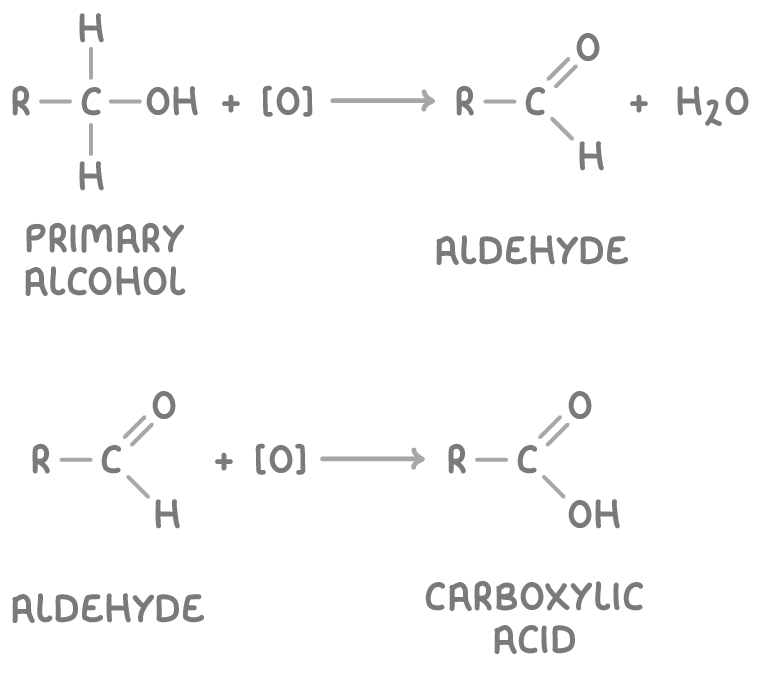
Where:
- [O] represents the oxidising agent.
- R is an alkyl group.
The orange dichromate(VI) ion (Cr2O72-) is reduced to the green chromium(III) ion (Cr3+) during these oxidation reactions.
Controlling alcohol oxidation to get aldehydes or acids
You can control how far primary alcohols are oxidised by using different reaction conditions:
- Gently heating the alcohol with acidified K2Cr2O7 in a distillation set-up stops at the aldehyde product.
- Refluxing the alcohol (or aldehyde) with acidified K2Cr2O7 continues the oxidation to the carboxylic acid.
Oxidising secondary alcohols to ketones
Ketones are produced when secondary alcohols are oxidised with acidified K2Cr2O7.
The oxidation reaction is:
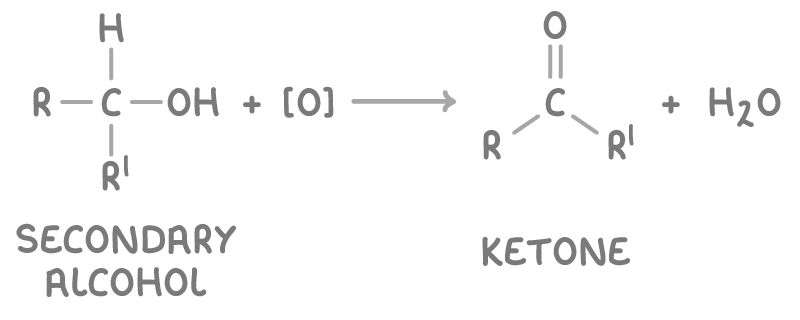
- [O] represents the oxidising agent.
- R and R’ represent alkyl groups.
Ketones cannot be oxidised further by acidified K2Cr2O7.