Reactions of Alkenes
This lesson covers:
- Electrophilic addition reactions of alkenes
- Addition of halogens to alkenes
- Hydration of alkenes
- Addition of hydrogen halides to symmetrical and unsymmetrical alkenes
- Addition of sulfuric acid to alkenes
Electrophilic addition reactions
Alkenes undergo a type of reaction called electrophilic addition across their carbon-carbon double bond. In this process:
- The π bond of the C=C double bond breaks.
- Atoms or groups add across the carbon atoms.
This happens because the C=C double bond is rich in electrons, making it a target for electrophiles - electron pair acceptors that are attracted to areas with a high electron density.
Common electrophiles that add to alkenes include:
- Polar molecules, where the positive δ+ atom is attracted to the C=C electrons, such as the Hδ+ of H-Br.
- Halogens such as chlorine and bromine.
Addition of halogens
Halogens react with alkenes in an electrophilic addition reaction to form dihalogenoalkanes.
The mechanism for addition of halogens to alkenes, using bromine and ethene as an example, is:
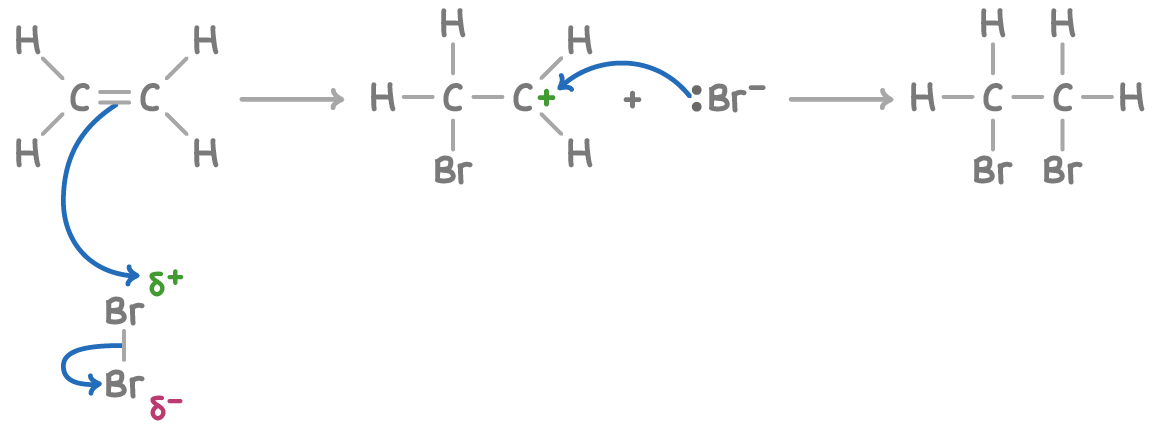
Step 1 - The high electron density of the C=C double bond repels electrons in the bromine molecule, polarising the Br-Br bond.
Step 2 - This leads to heterolytic fission of the Br-Br bond, with one bromine atom taking the bonding pair of electrons, creating a positively charged bromine which bonds to one of the carbon atoms.
Step 3 - A positively charged carbocation intermediate forms. The negatively charged bromide ion then bonds to the other carbon atom.
Step 4 - The final product, 1,2-dibromoethane, has a bromine atom bonded to each of the original alkene carbon atoms.
The overall equation for this reaction is:
H2C=CH2 + Br2 ➔ BrCH2CH2Br
Bromine water tests for carbon-carbon double bonds
The reaction of bromine with alkenes is a useful test for C=C bonds.
Bromine water, an orange solution of bromine, reacts with an alkene:
- The alkene reacts with bromine via electrophilic addition.
- This reaction removes the orange colour as the bromine is consumed.
- The product is a colourless dibromoalkane solution.
The rapid decolourisation of orange bromine water indicates the presence of carbon-carbon double bonds, offering a simple test for alkenes.
Hydration to form alcohols
Under the influence of a phosphoric(V) acid catalyst, alkenes react with steam at 300°C and 60-70 atm pressure, undergoing hydration to form alcohols.
For example, ethene is hydrated to produce ethanol:
H2C=CH2(g) + H2O(g) ⇌ CH3CH2OH(g)
The reaction mechanism is:
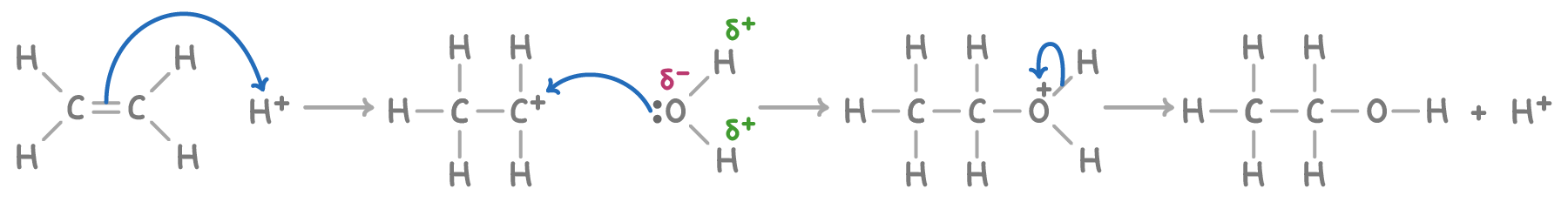
This reaction is significant in the industrial production of ethanol from ethene.
A limitation is the reaction's reversible nature, with only about 5% yield. However, recycling unreacted ethene can increase overall yields to over 95%.
Addition of hydrogen halides
Hydrogen halides like HCl and HBr add across the C=C double bond in an electrophilic addition, forming halogenoalkanes.
In symmetrical alkenes, the double bonded carbons are identical, leading to a single monosubstituted product.
For example, ethene reacts with HBr to form bromoethane:
H2C=CH2 + HBr ➔ CH3CH2Br
In unsymmetrical alkenes with two different carbon groups, hydrogen halides can form two positional isomers.
For example, propene reacts with HBr to form a mixture of 1-bromopropane and 2-bromopropane:
CH3CH=CH2 + HBr ➔ CH3CH2CH2Br (1-bromopropane)
CH3CH=CH2 + HBr ➔ CH3CHBrCH3 (2-bromopropane)
The ratio of these products depends on the stability of the intermediate carbocation.
Predicting major addition products
The major product in hydrogen halide addition to unsymmetrical alkenes can be predicted by carbocation stability.
Carbocation stability increases with more alkyl groups
The reaction generates a positively charged carbocation intermediate. Its stability varies based on its structure:
- Primary carbocations - These have one alkyl group attached to the positively charged carbon. These are the least stable.
- Secondary carbocations - These have two alkyl groups at the carbocation centre, offering moderate stability.
- Tertiary carbocations - These have three alkyl groups are the most stable.
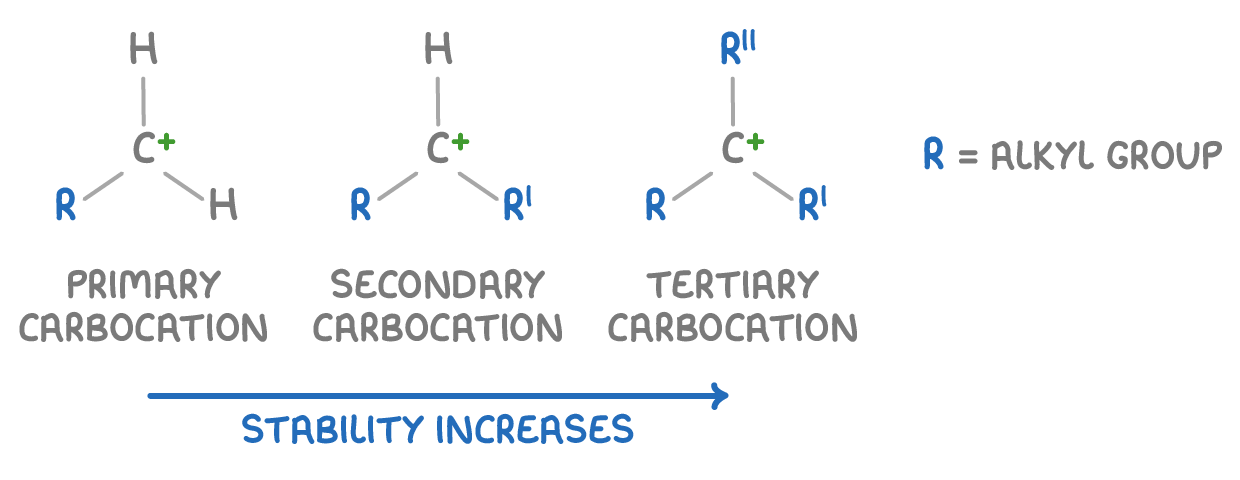
Alkyl groups stabilise the positive charge through inductive effects, with more groups providing greater stability.
Most stable carbocation forms major product
The major halogenoalkane product forms via the most stable carbocation intermediate.
For example, in the reaction of propene with HBr, the secondary carbocation forms preferentially, making 2-bromopropane the major product.
Worked example 1 - Determining the major addition product
Predict the major product when 2-methylbut-2-ene reacts with HCl.
Step 1: Determine structure of 2-methylbut-2-ene
2-methylbut-2-ene: (CH3)2C=CHCH3
Step 2: Determine potential carbocation intermediates
When HCl adds to 2-methylbut-2-ene, two types of carbocations can form:
- Carbocation at C2: (CH3)2C+CH2CH3 (tertiary carbocation)
- Carbocation at C3: (CH3)2CHC+H(CH3) (secondary carbocation)
Step 3: Determine the most stable carbocation
Tertiary carbocations (like the one at C2) are more stable than secondary carbocations (like the one at C3) due to greater alkyl group substitution.
Therefore, the major product will be formed via the more stable carbocation. In this case, it's the tertiary carbocation at C2.
Step 4: Predict major product
The major product of the addition of HCl to 2-methylbut-2-ene is 2-chloro-2-methylbutane:
(CH3)2C=CHCH3 + HCl ➔ (CH3)2C(Cl)CH2CH3
Addition of concentrated sulfuric acid
Cold concentrated H2SO4 adds across the C=C bond in an electrophilic addition reaction, initially forming alkyl hydrogen sulfates.
For example, ethene reacts with sulfuric acid to form ethyl hydrogen sulfate:
H2C=CH2 + H2SO4 ➔ CH3CH2OSO3H
Water and heat hydrolyse the alkyl hydrogen sulfate back to alcohols and sulfuric acid.
For example, ethyl hydrogen sulfate hydrolyses to ethanol and sulfuric acid:
CH3CH2OSO3H + H2O ➔ CH3CH2OH + H2SO4
Reaction with unsymmetrical alkenes leads to a mix of alcohol products, with the major product again coming through the most stable carbocation.