Developing Ideas of the Atom
This lesson covers:
- Why atomic models have changed over time
- The solid sphere model
- The plum pudding model
- The nuclear model
- The Bohr model
Accepted atomic models have evolved with new evidence
Our understanding of the atom's structure has grown considerably over the past few centuries. As scientists have discovered new experimental evidence, they've needed to revise their atomic models to more accurately explain their findings.
It's crucial to recognise that the currently accepted model is still just a model - a simplified depiction of reality meant to help us understand chemical phenomena.
Early atomic models
- Dalton's solid sphere model (early 19th century)
- Proposed by John Dalton, this model suggested that atoms were indivisible, solid spheres.
- It also suggested that different elements were made up of different types of these spheres.
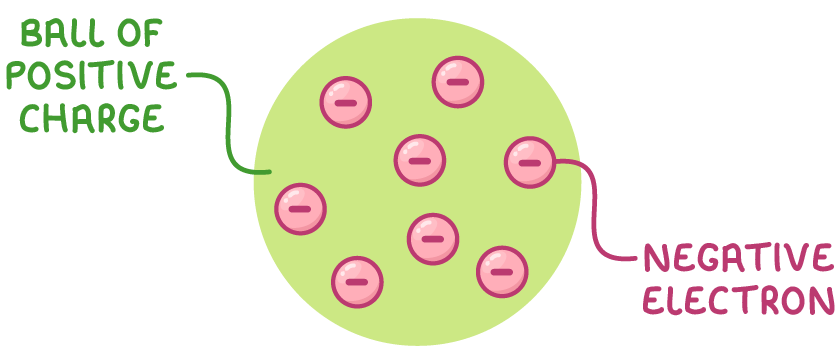
2. Thomson's plum pudding model (1897)
- The discovery of the electron by J.J. Thomson proved that atoms were divisible.
- His 'plum pudding' model depicted atoms as a positively charged 'pudding' with negative electrons embedded throughout, similar to the raisins in a plum pudding.
Rutherford's gold foil experiment and the nuclear model
The year 1909 saw Ernest Rutherford, along with his students Hans Geiger and Ernest Marsden, perform a groundbreaking experiment that would redefine atomic theory.
The experiment:
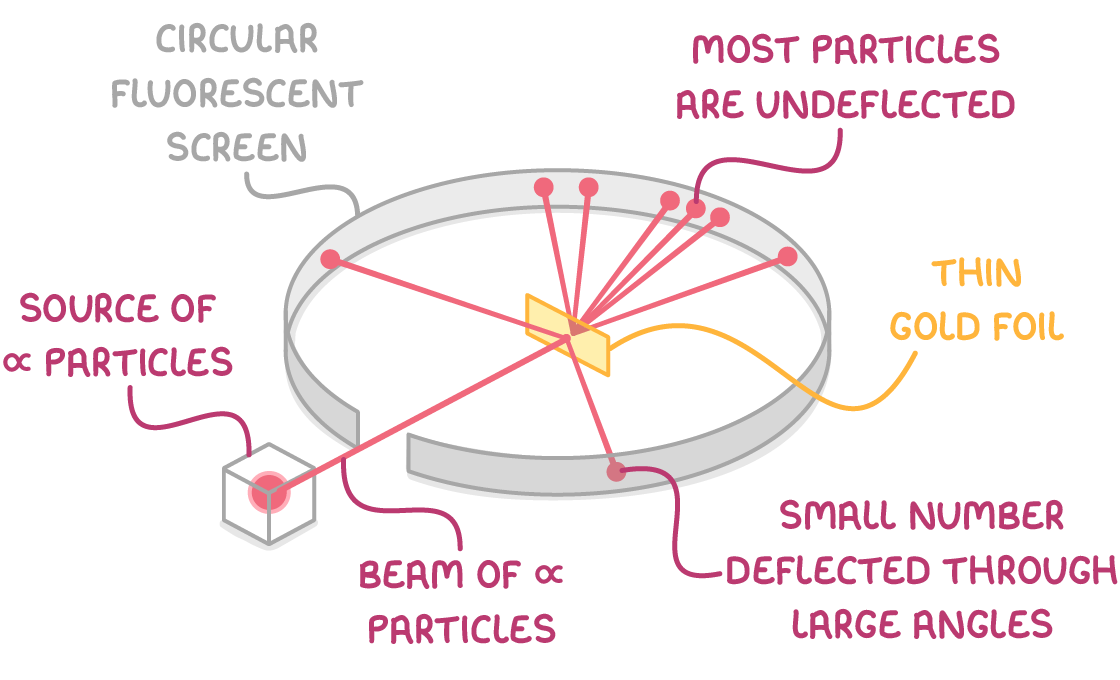
- Involved firing alpha particles (which are positively charged) at a sheet of thin gold foil inside a vacuum.
- According to the plum pudding model, the positive 'pudding' would slightly deflect most of the particles.
- Contrary to expectations, most alpha particles passed straight through, with a few deflecting at large angles or even bouncing back.
Rutherford's conclusions:
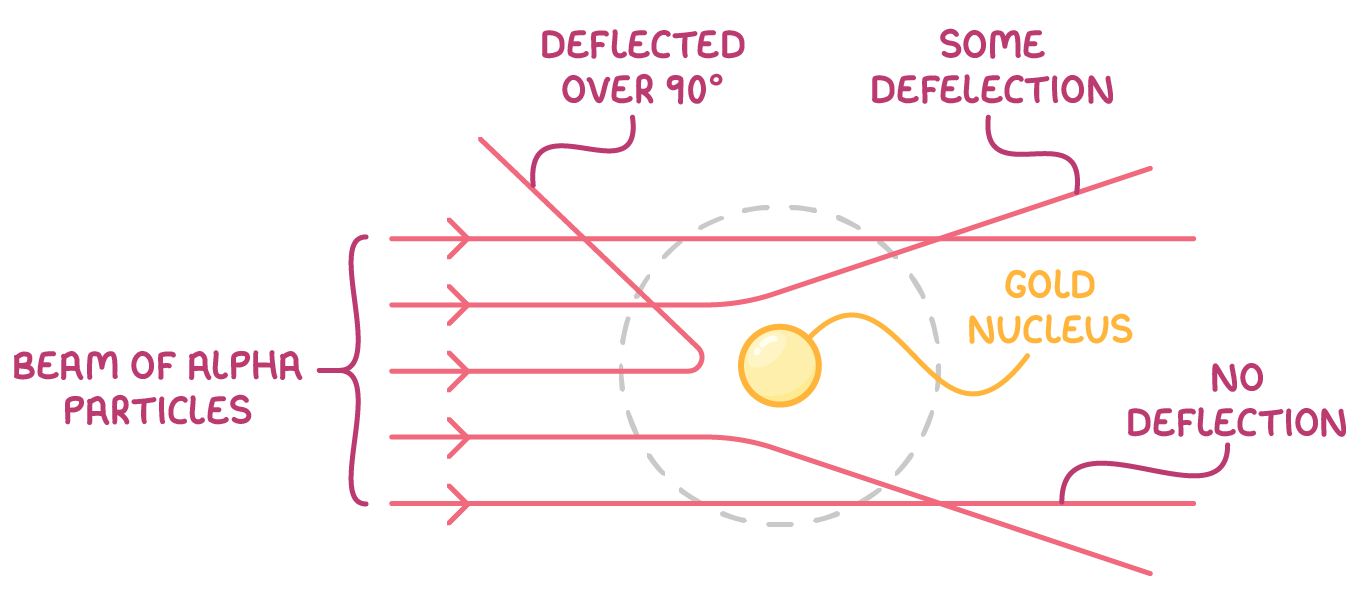
- The fact that most alpha particles passed through the gold foil undeflected indicated that atoms are mostly empty space.
- The small number of particles that deflected at large angles or bounced back suggested the presence of a tiny, dense, positively charged nucleus at the centre of the atom. This nucleus was responsible for the strong deflection of the positively charged alpha particles due to electrostatic repulsion.
- Since the atom was mostly empty space with a dense nucleus, Rutherford proposed that negative electrons must orbit the nucleus in a 'cloud' to maintain the atom's overall neutrality.
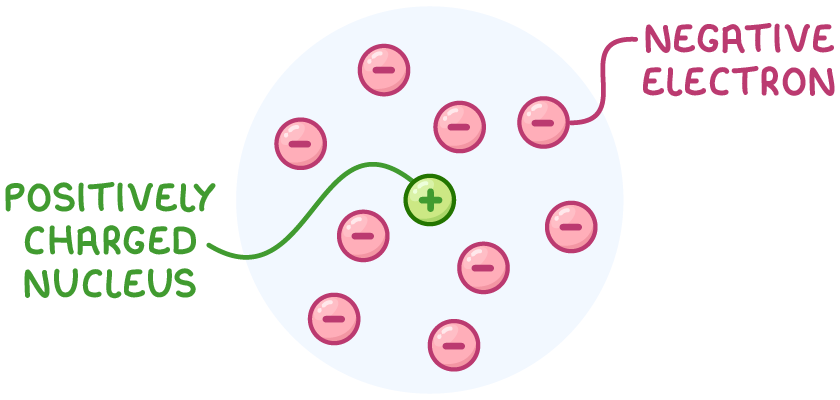
This discovery, based on the surprising results of the gold foil experiment, gave birth to the nuclear model of the atom.
Modifications to Rutherford's nuclear model
Rutherford's nuclear model was a significant step forward but required adjustments as further evidence came to light:
Discovery of the proton
- Research by Henry Moseley showed that the nuclear charge increases by one unit from one element to the next.
- This insight led Rutherford to identify positively charged particles within the nucleus, which he named protons.
- The differences in nuclear charges among elements could now be explained by the presence of varying numbers of protons.
Prediction and discovery of the neutron
- A remaining mystery was why atomic nuclei were heavier than they should be if they contained only protons.
- Rutherford speculated about the existence of another type of nuclear particle.
- James Chadwick later discovered the neutron, which provided the mass needed to account for the discrepancy in the nucleus's weight.
Bohr's model and the introduction of electron shells
A flaw in Rutherford's model was its inability to explain why electrons, attracted to the nucleus, wouldn't simply collapse into it.
To solve this, Niels Bohr suggested a model in which:
- Electrons orbit the nucleus in specific energy levels, or shells.
- They can jump between these shells by absorbing or emitting light of particular wavelengths.
This model accounted for the observed spectra of light emitted or absorbed by atoms, a phenomenon Rutherford's model could not explain.
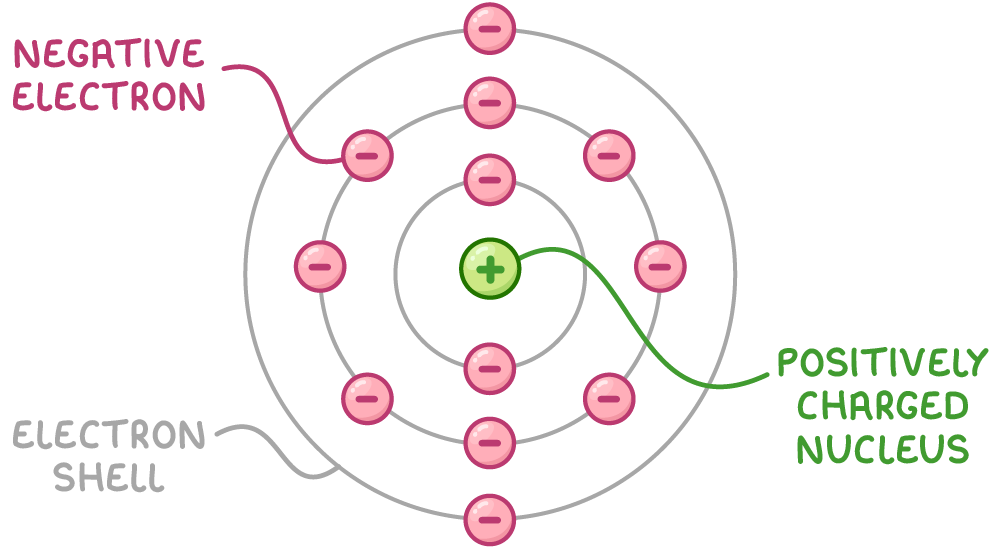
Refining the Bohr model with subshells
Subsequent experiments revealed that electrons within the same shell could possess different energy levels, leading to the introduction of subshells in the Bohr model.
While even more accurate atomic models exist today, the refined Bohr model remains useful due to its simplicity and ability to explain many chemical concepts, such as trends in bonding and ionisation energy.