Complex Formation and the Shape of Complex Ions
This lesson covers:
- What complex ions are
- How complex ions are represented
- Common coordination numbers and shapes
- Types of ligand
- How to determine oxidation states
Defining complex ions
A complex ion is a central metal atom or ion surrounded by coordinately bonded ligands.
- A ligand is an atom, ion or molecule that donates a pair of electrons to the central metal ion.
- A coordinate bond (or dative covalent bond) is a covalent bond in which bond electrons in the shared pair come from same atom or ion.
An example of a complex ion is [Cr(H2O)6]3+; the oxygen atom of each water molecule donates a lone pair of electrons to the central Cr3+ ion:
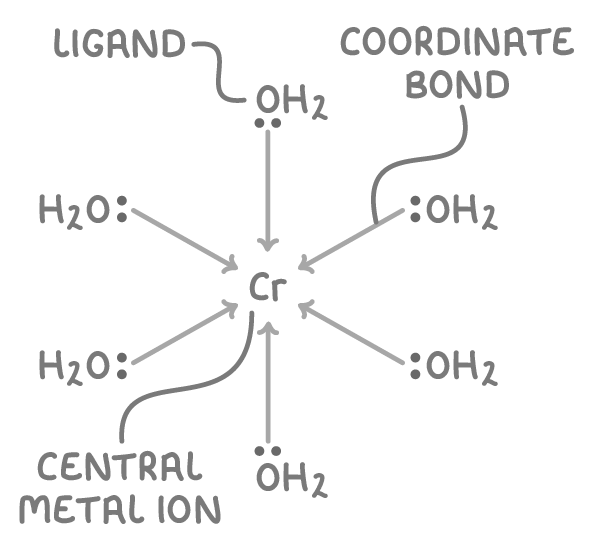
Transition metal elements form complex ions because their partially filled, energetically accessible d-orbitals can accept lone pairs of electrons from ligands.
Representing complex ions
Complex ions are enclosed inside square brackets, with the overall charge of the complex shown outside the brackets.
Ligands are often represented inside round brackets, with the number of ligands shown outside the brackets.
For example, the complex ion [Cu(NH3)4]2+ has:
- A central Cu ion
- Four NH3 ligands
- An overall charge of +2
Common coordination numbers and shapes
The number of coordinate bonds formed between the ligands and metal ion is called the coordination number.
Complex ions usually have coordination numbers of 6 or 4.
Some examples of complex ions with different coordination numbers are shown below:
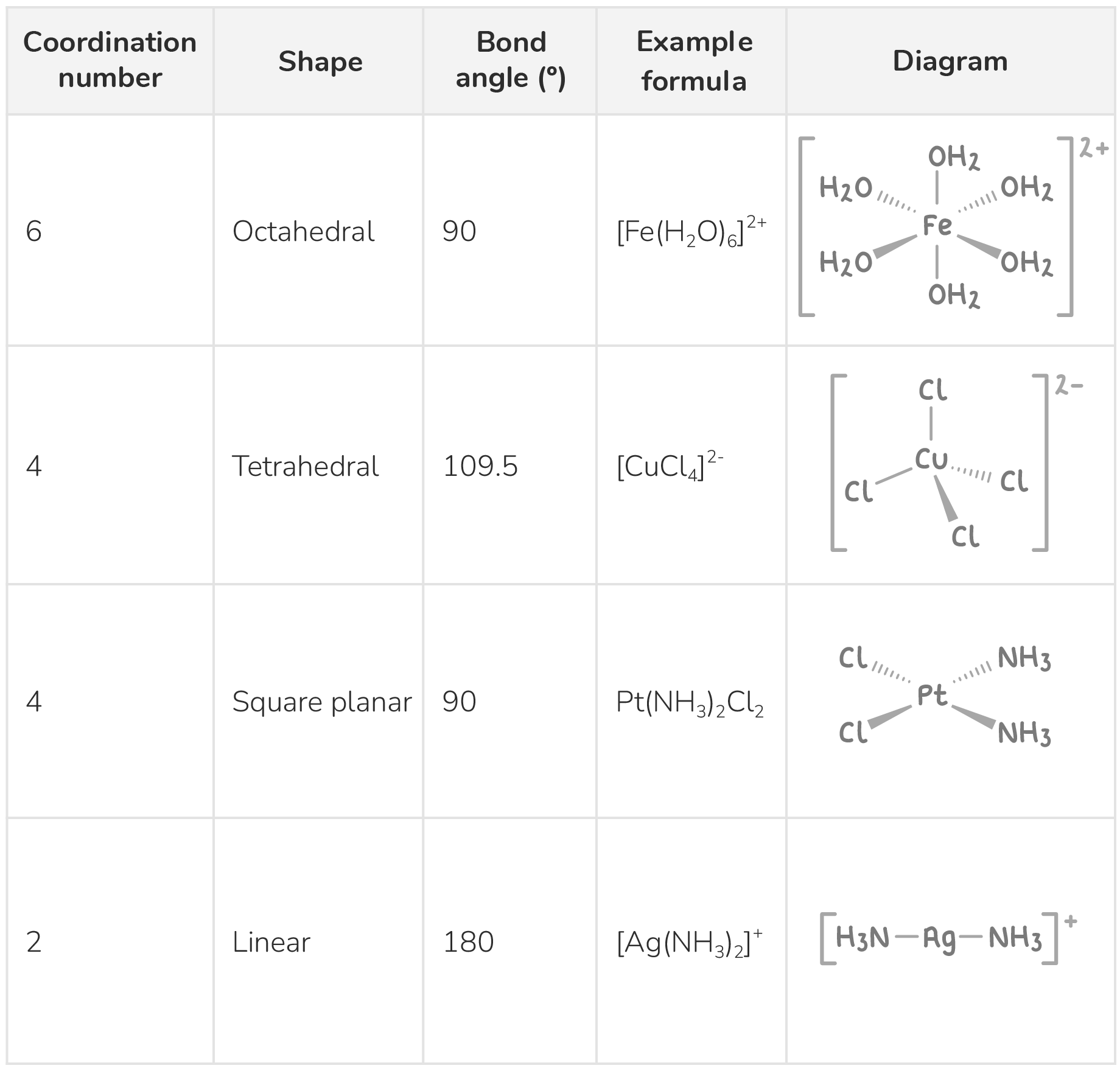
The complex ion [Fe(H2O)6]2+ has a coordination number of 6 as the small water ligands can pack tightly around the central metal ion, while [CuCl4]2- only has a coordination number of 4 as the chloride ligands are larger, limiting the number that can fit around the copper ion.
Types of ligand
Ligands can be categorised by how many coordinate bonds they are capable of forming with metal ions:
- Monodentate ligands - These have one lone electron pair available for bonding. Examples include H2O, NH3, Cl-, and CN-.
- Multidentate ligands - These have two or more lone electron pairs available for bonding. Examples include the bidentate ligands 1,2-diaminoethane (en) and ethanedioate.
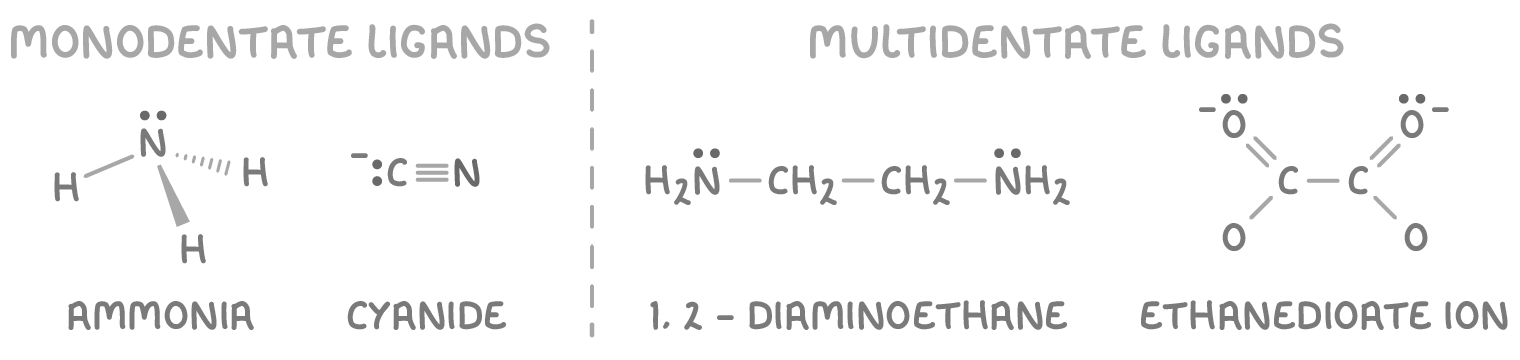
Ligands can also be categorised by their overall charge:
- Neutral ligands - These are molecules or atoms such as H2O, NH3, and en.
- Negatively charged ligands - These are anions such as Cl-, CN-, and OH-.
Determining oxidation states
The overall charge on a complex ion is also called the total oxidation state.
The equation is calculate the oxidation state of the central metal cation is:
Oxidation state of metal cation = Total oxidation state− Sum of oxidation states of all ligands
Worked example 1 - Calculating the oxidation state of a metal cation
Determine the oxidation state of the central metal cation in the complex ion [Cu(NH3)4]2+.
Step 1: Calculate the total oxidation state
The complex ion [Cu(NH3)4]2+ has an overall charge of +2. Therefore, the total oxidation state is +2.
Step 2: Calculate the sum of oxidation states of all ligands
Each ammonia (NH3) ligand is neutral and has an oxidation state of 0.
There are 4 ammonia ligands, so the sum of oxidation states of all ligands is 0×4=0
Step 3: Equation
Oxidation state of metal cation = Total oxidation state− Sum of oxidation states of all ligands
Step 4: Substitution and correct evaluation
Oxidation state of metal cation =+2−0=+2
So, the oxidation state of the central copper (Cu) cation in the complex ion [Cu(NH3)4]2+ is +2.
Worked example 2 - Calculating the oxidation state of a metal cation
Determine the oxidation state of the central metal cation in the complex ion [Cr(H2O)4Cl2]+.
Step 1: Calculate the total oxidation state
The complex ion [Cr(H2O)4Cl2]+ has an overall charge of +1. Therefore, the total oxidation state is +1.
Step 2: Calculate the sum of oxidation states of all ligands
Each water (H2O) ligand is neutral and has an oxidation state of 0.
Each chloride (Cl) ligand is charged and has an oxidation state of -1.
There are 4 water ligands and 2 chloride ligands, so the sum of oxidation states of all ligands is (0×4)+(−1×2)=−2
Step 3: Equation
Oxidation state of metal cation = Total oxidation state− Sum of oxidation states of all ligands
Step 4: Substitution and correct evaluation
Oxidation state of metal cation =+1−(−2)=+3
So, the oxidation state of the central chromium (Cr) cation in the complex ion [Cr(H2O)4Cl2]+ is +3.