The Periodic Table
This lesson covers:
- The arrangement of the modern periodic table into blocks, periods and groups
- How to use the periodic table to determine the electron configuration of elements
The modern periodic table
The modern periodic table arranges elements in order of increasing atomic (proton) number. This arrangement reveals patterns in the properties of elements, allowing chemists to make predictions about their behavior.
Periodic table structure
The periodic table is organised into periods (rows) and groups (columns):
- Periods: Elements in the same period have the same number of electron shells. As you move down a period, the number of electron shells increases.
- Groups: Elements in the same group have similar electron configurations in their outer shell. As a result, they exhibit similar physical and chemical properties.
The periodic table can be further divided into three blocks based on the type of subshell being filled in the outermost electron shell:
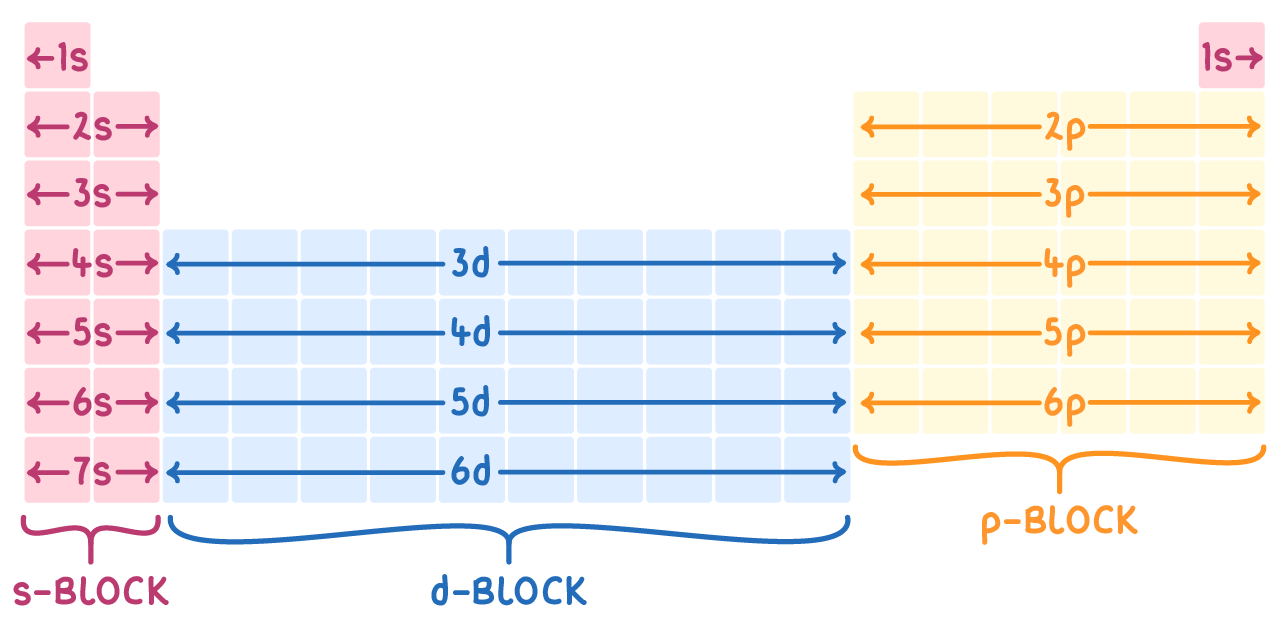
- s-block: Elements in the s-block have their outermost electrons in an s orbital.
For example, the electronic configuration of lithium (Li) is 1s2 2s1.
- p-block: Elements in the p-block have their outermost electrons in an p orbital.
For example, the electronic configuration of oxygen (O) is 1s2 2s2 2p4.
- d-block: Elements in the d-block have their outermost electron in a d orbital.
For example, the electronic configuration of iron (Fe) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
Determining electron configurations
You can use the table to deduce the electron configuration of elements:
- Identify the period - This shows how many electron shells
- Note which block it falls in - This shows which subshell is being filled in the outer electron shell.
- Write out the complete configuration by building up from the 1s orbital
Worked example 1 - Determining the electron configuration of vanadium
Write the electronic configuration of an atom of vanadium, V.
Step 1: Identify which period vanadium belongs to
Vanadium is located in period 4, so it has 4 electron shells.
Step 2: Identify which block vanadium belongs to
Vanadium is part of the d-block, so its outer shell includes a partially filled d subshell.
Step 3: Write the complete electronic configuration
The complete configuration is:
Period 1: 1s2
Period 2: 2s2 2p6
Period 3: 3s2 3p6
Period 4: 4s2 3d3
Therefore, the electron configuration for vanadium is 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
The abbreviated configuration is [Ar] 4s2 3d3.