Year 2 Synthetic Routes
This lesson covers:
- How chemists aim to design safe and efficient synthesis routes
- Summaries of key reactions for aliphatic compounds
- Summaries of key reactions for aromatic compounds
- How functional groups determine reactivity
Chemists aim for safe, efficient and cost-effective syntheses
When designing organic syntheses, chemists aim to ensure greener, more sustainable, and economically viable processes. They do this by focusing on the following key aspects:
Safety:
Chemists work to reduce the risk of accidents and environmental damage by using non-hazardous starting materials whenever possible. They also design processes that do not require solvents, particularly flammable or toxic ones, to decrease the potential for harm and environmental impact.
Efficiency:
To maximise yield while minimising waste, chemists pursue high percentage yield and atom economy. This ensures that the majority of the reactants end up in the desired product, reducing waste. They also design production methods with fewer reaction steps to minimise the use of resources and the generation of byproducts.
Cost-effectiveness:
By using non-hazardous starting materials, avoiding the use of solvents, and designing processes with fewer steps, chemists can significantly reduce the overall cost of the synthesis. This makes the process more economically viable and sustainable in the long run.
Summary of key organic reactions for aliphatic compounds
Below is an overview of the primary types of organic reactions we've discussed for aliphatic compounds.
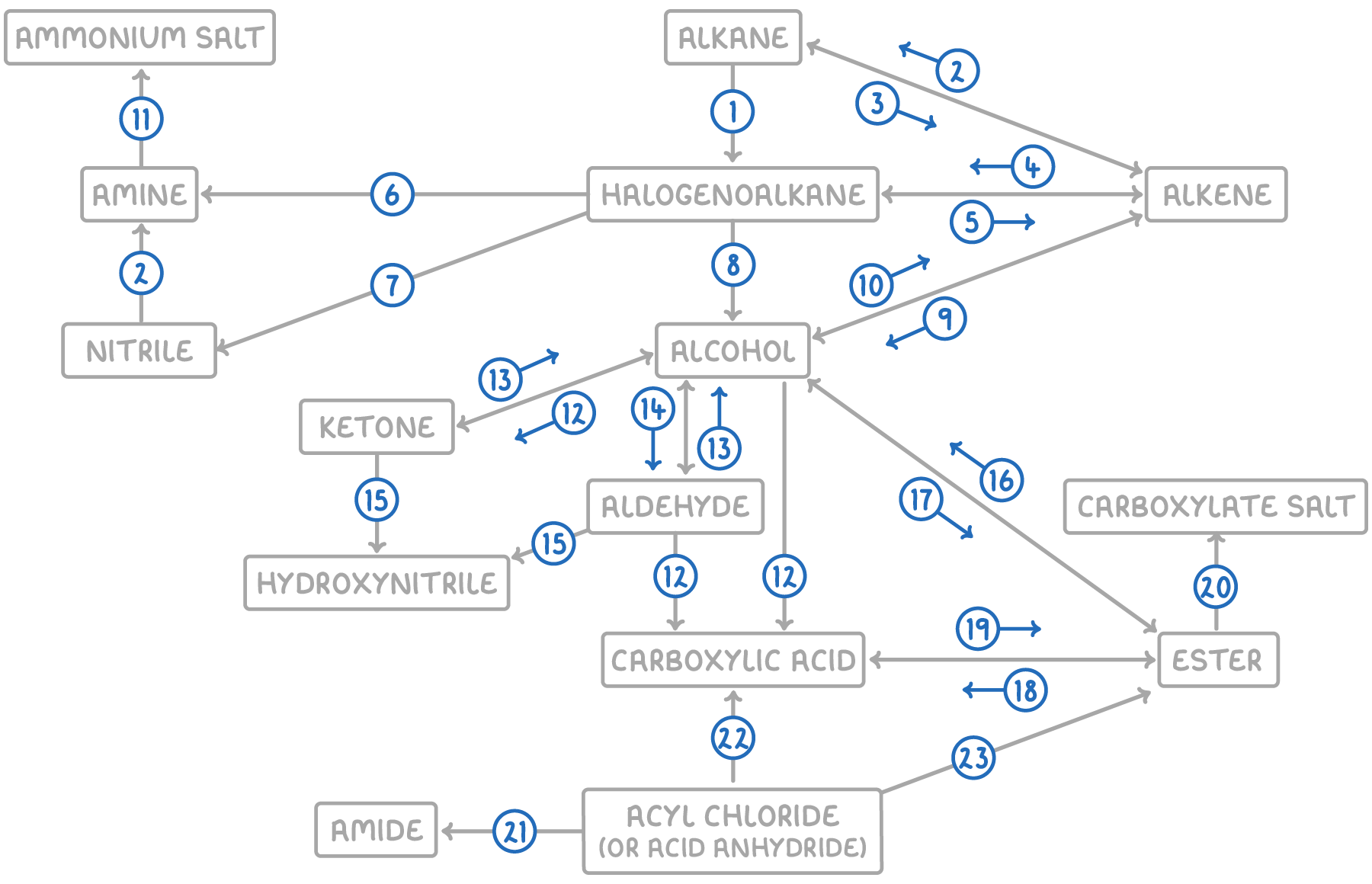
| Reaction | Reagent and conditions | Reaction type |
|---|---|---|
| 1 | Halogen, UV light | Free radical substitution |
| 2 | H2, nickel catalyst, 150°C | Electrophilic addition |
| 3 | Zeolite catalyst, heat | Thermal decomposition |
| 4 | Hydrogen halide or halogen | Electrophilic addition |
| 5 | KOH in ethanol, reflux | Elimination |
| 6 | Conc. NH3 in ethanol, heat under pressure | Nucleophilic substitution |
| 7 | KCN in ethanol, reflux | Nucleophilic substitution |
| 8 | NaOH(aq), reflux | Nucleophilic substitution |
| 9 | Steam, H3PO4 catalyst, 300°C, 60 atm | Electrophilic addition |
| 10 | Conc. H2SO4 catalyst, heat | Elimination |
| 11 | Dilute HCl(aq) OR halogenoalkane, heat | Acid-base OR nucleophilic substitution |
| 12 | K2Cr2O7(aq), H2SO4 catalyst, reflux | Oxidation |
| 13 | NaBH4(aq), heat | Nucleophilic addition |
| 14 | K2Cr2O7(aq), H2SO4 catalyst, distill | Oxidation |
| 15 | NaCN(aq), H2SO4 catalyst | Nucleophilic addition |
| 16 | Dilute HCl(aq) or dilute NaOH(aq), reflux | Hydrolysis |
| 17 | Carboxylic acid, conc. H2SO4 OR acid anhydride | Condensation |
| 18 | Dilute HCl(aq), heat | Hydrolysis |
| 19 | Alcohol, conc. H2SO4 | Condensation |
| 20 | Dilute NaOH(aq) | Acid-base |
| 21 | NH3 or amine | Nucleophilic addition-elimination |
| 22 | H2O | Nucleophilic addition-elimination |
| 23 | Alcohol | Nucleophilic addition-elimination |
Summary of key organic reactions for aromatic compounds
Below is an overview of the primary types of organic reactions we've discussed for aromatic compounds.
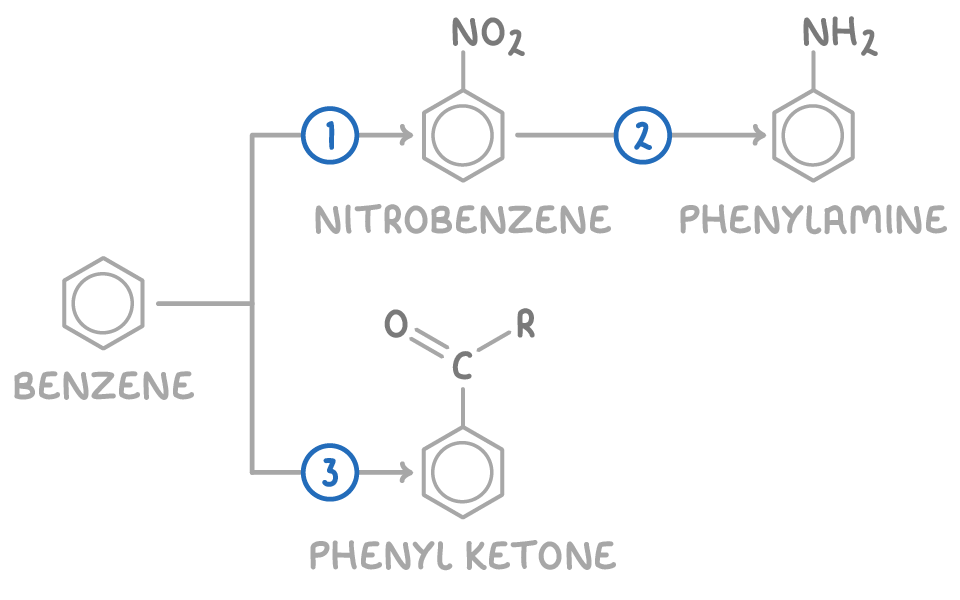
| Reaction | Reagent and conditions | Reaction type |
|---|---|---|
| 1 | Conc. HNO3, conc. HCl, 25-60°C | Electrophilic substitution |
| 2 | Sn, conc. HCl, reflux, then NaOH(aq) | Reduction |
| 3 | RCOCl, AlCl3 catalyst, heat | Friedel-Crafts acylation |
Importance of functional groups
Functional groups are responsible for a molecule's chemical properties and reactivity. Organic molecules are categorised by homologous series based on their functional groups.
Some properties and typical reactions associated with key functional groups are:
| Homologous series | Functional group | Properties | Typical reactions |
|---|---|---|---|
| Halogenoalkane | C-X | Polar bond | Nucleophilic substitution, elimination |
| Aldehyde/Ketone | C=O | Polar C=O bond | Oxidation (aldehydes only), reduction, nucleophilic addition |
| Nitrile | C≡N | Electron deficient C | Reduction |
| Hydroxynitrile | R2C(OH)C≡N | Electron deficient C | Reduction |
| Carboxylic acid | -COOH | Electron deficient C | Neutralisation, condensation |
| Ester | RCOOR' | Electron deficient C | Hydrolysis |
| Amine | C–NR2 | Lone pair on nitrogen is basic and can act as a nucleophile | Neutralisation, nucleophilic substitution |
| Amide | RCONHR’ | Electron deficient C | Hydrolysis |
| Aromatic compounds | C6H5- | Stable delocalised ring of electrons | Electrophilic substitution |
| Acyl chloride | -COCl | Electron deficient C | Nucleophilic addition-elimination, Friedel-Crafts acylation |
| Acid anhydride | RCOOCOR' | Electron deficient C | Condensation |
So by recognising functional groups, organic chemists can predict the likely behaviour and reactivity of compounds. This allows them to design effective syntheses.