Metallic Bonding
This lesson covers:
- The structure of metals
- How metallic bonds are formed
- Factors affecting the strength of metallic bonds
- The properties of metals
Metals have metallic lattice structures
Metals exist as giant lattice structures made up of:
- Positively charged metal cations (e.g. Na+, Mg2+)
- Delocalised electrons that move freely between the cations
The outer electrons of metal atoms leave to become delocalised, resulting in positively charged metal cations.
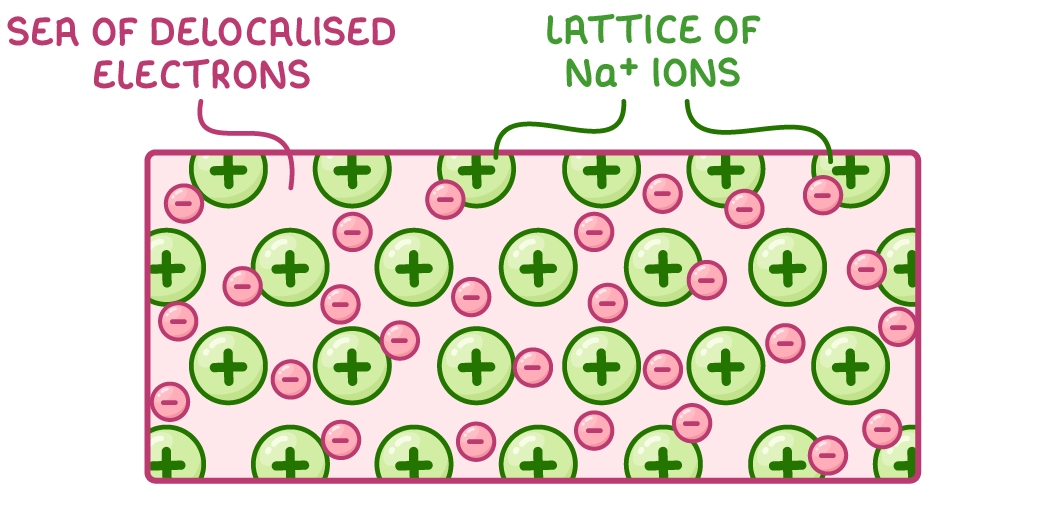
A 2D model of sodium’s giant metallic lattice structure is shown above.
Metallic bonding occurs between metal cations and delocalised electrons
- Metallic bonding refers to the electrostatic attractions between positively charged metal ions (e.g. Na+, Mg2+) and negatively charged delocalised electrons.
- These metallic bonds are very strong and hold the metals in their lattice structure.
Strength of metallic bonds
The strength of metallic bonds determines the melting point of a metal. The stronger the metallic bonds, the higher the melting point.
The strength of these bonds is influenced by three main factors:
- Number of delocalised electrons per atom:
- Metals with a higher number of delocalised electrons per atom tend to form stronger metallic bonds.
- The increased number of delocalised electrons allows for stronger electrostatic attractions between the electrons and the metal cations.
- Charge of the metal cation:
- A higher cation charge results in stronger electrostatic attractions between the cation and the delocalised electrons.
- Radius of the metal cation:
- Smaller metal cations have a higher charge density, which allows them to hold the delocalised electrons closer to the nucleus.
- This proximity enhances the electrostatic attractions between the cation and the electrons, resulting in a stronger metallic bond.
Magnesium has a higher melting point than sodium
To illustrate these concepts, let's compare the melting points of magnesium and sodium:
Magnesium has a melting point of 650°C, while sodium has a melting point of 98°C.
Magnesium has a higher melting point than sodium because:
1. Each magnesium atom donates two electrons to the delocalised electron sea, whereas each sodium atom donates only one electron.
2. Magnesium has a +2 charge, while sodium has a +1 charge.
3. Magnesium cations (Mg2+) are smaller than sodium cations (Na+).
Properties explained by metallic bonding
The properties of metals result from their metallic bonding and lattice structure:
- High melting and boiling points - The strong electrostatic forces of attraction between the positively charged metal cations and the sea of delocalised electrons must be overcome for the lattice to break apart; this requires large amounts of energy.
- Good conductors of electricity and heat - The delocalised electrons can flow freely through the lattice to transfer charge and heat energy.
- Malleable and ductile - Layers of the cation lattice can slide over one another when the metal is hammered or pulled because there are no bonds locking individual cations together.
- Insoluble - The strength of the metallic bonding prevents water or other solvent molecules from pulling the cations away from the lattice structure and dissolving the metal.