Proton NMR
This lesson covers:
- What 1H NMR spectroscopy tells us
- Chemical shifts and functional group identification
- Peak splitting patterns and the n+1 rule
- The role of deuterated solvents in 1H NMR
1H NMR determines hydrogen environments
Proton nuclear magnetic resonance (1H NMR) spectroscopy is a technique that provides detailed information about the different environments of hydrogen atoms within an organic molecule.
In a 1H NMR spectrum:
- Each peak represents a set of chemically equivalent hydrogen atoms, which is referred to as a hydrogen environment.
- The peak area is proportional to the number of hydrogen atoms in that environment.
- The position of the peak on the chemical shift scale helps identify the type of hydrogen atom present.
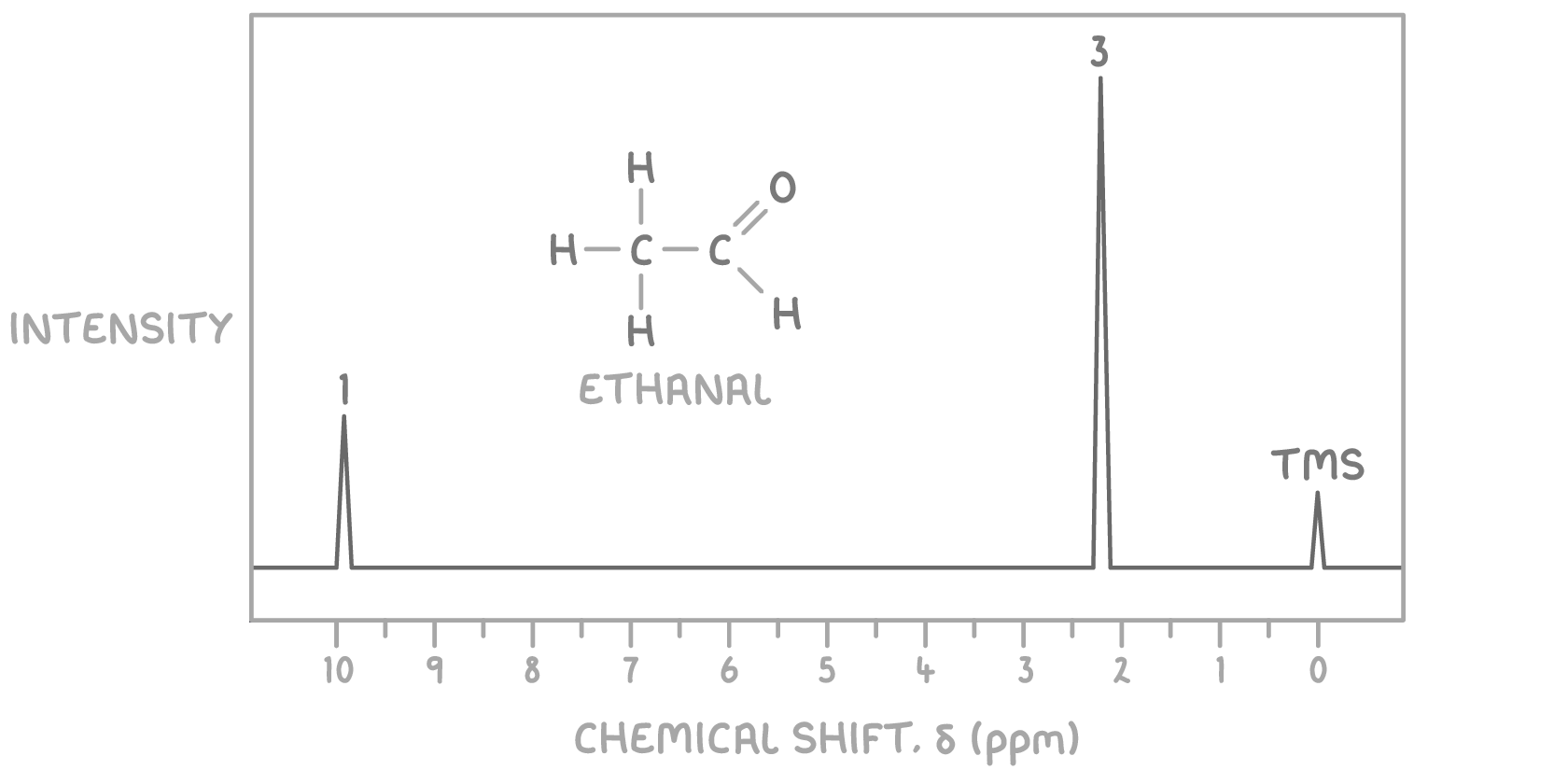
For example, the 1H NMR spectrum of ethanal, shown above, displays:
- 2 peaks, indicating two different environments for the protons.
- Peak areas in a 3:1 ratio, corresponding to the number of protons in each environment.
Chemical shift ranges help assign peaks
The chemical shift (δ) value of each peak specifies its exact position.
This value, measured in parts per million (ppm), can help identify the hydrogen environment:
| Chemical Shift δ (ppm) | Type of H atom |
|---|---|
| 0.5 – 5.0 | ROH (alcohol) |
| 0.7 – 1.2 | RCH3 |
| 1.0 – 4.5 | RNH2 (amine) |
| 1.2 – 1.4 | R2CH2 |
| 1.4 – 1.6 | R3CH |
| 2.1 – 2.6 | R-(C=O)-CH (ketone) |
| 3.1 – 3.9 | R-O-CH |
| 3.1 – 4.2 | RCH2Br or RCH2Cl |
| 3.7 – 4.1 | R-(C=O)-O-CH (ester) |
| 4.5 – 6.0 | R-C=CH (alkene) |
| 9.0 – 10.0 | R-(C=O)-H (aldehyde) |
| 10.0 – 12.0 | R-(C=O)-OH (carboxylic acid) |
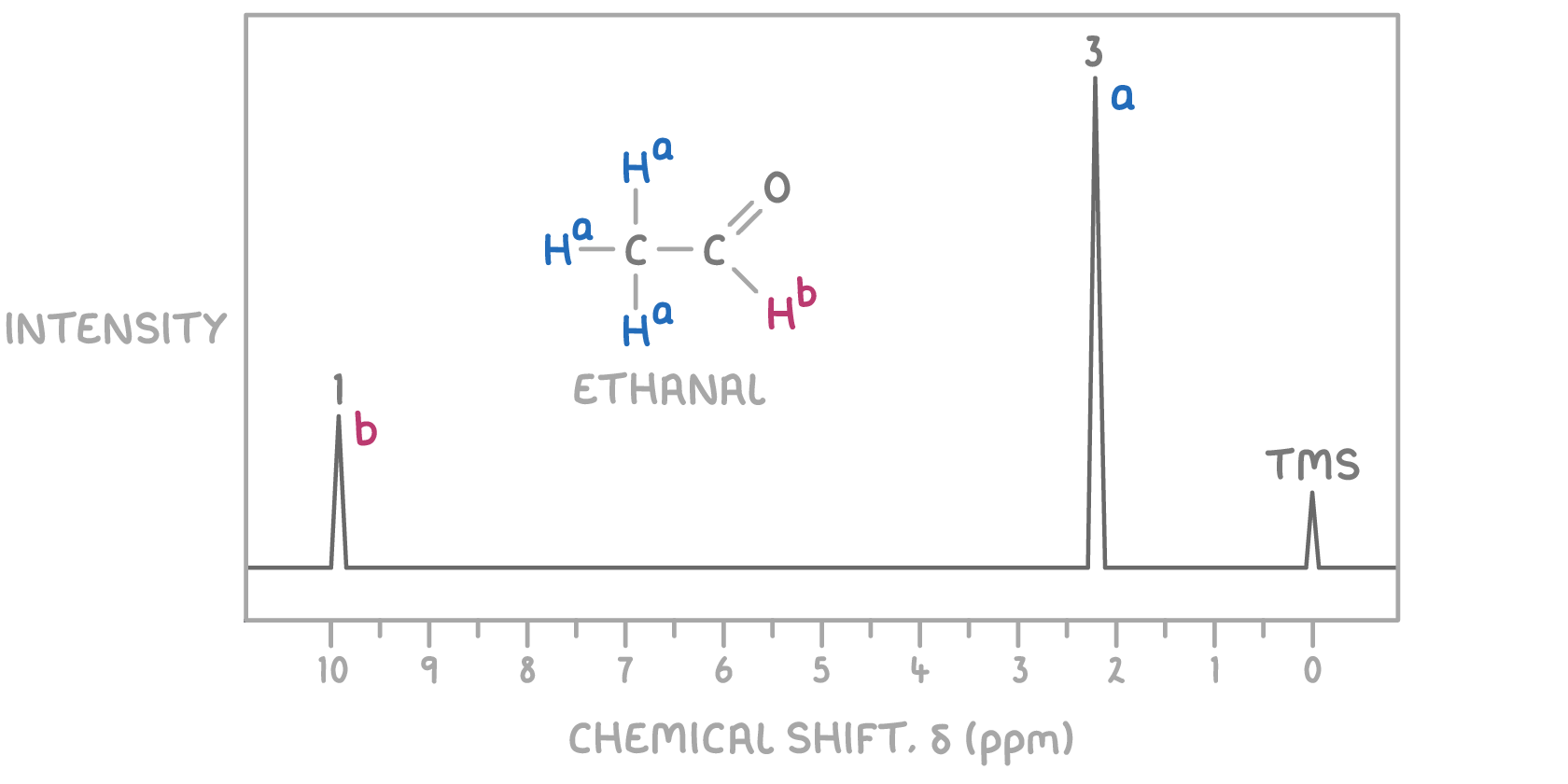
For example, in the 1H NMR spectrum of ethanal shown above:
- The peak at δ 2.2 ppm is indicative of CH3-, aligning with CH3CHO
- The peak at δ 9.8 ppm corresponds to -CHO, further confirming CH3CHO
This helps verify the structure through the spectrum.
Spin-spin coupling
Spin-spin coupling among adjacent hydrogen atoms results in the splitting of NMR peaks into sub-peaks. This effect occurs only between hydrogens on neighbouring carbons. Spin-spin coupling patterns reveal the number of non-equivalent protons on adjacent carbon atoms.
The formula for determining the number of peaks in a split signal is the n+1 rule:
For a proton with n protons attached to an adjacent carbon atom, the number of sub-peaks in a splitting pattern = n + 1
This rule adds another layer of structural detail:
| Peak type | Number of neighbouring hydrogens |
|---|---|
| Singlet | 0 |
| Doublet | 1 |
| Triplet | 2 |
| Quartet | 3 |
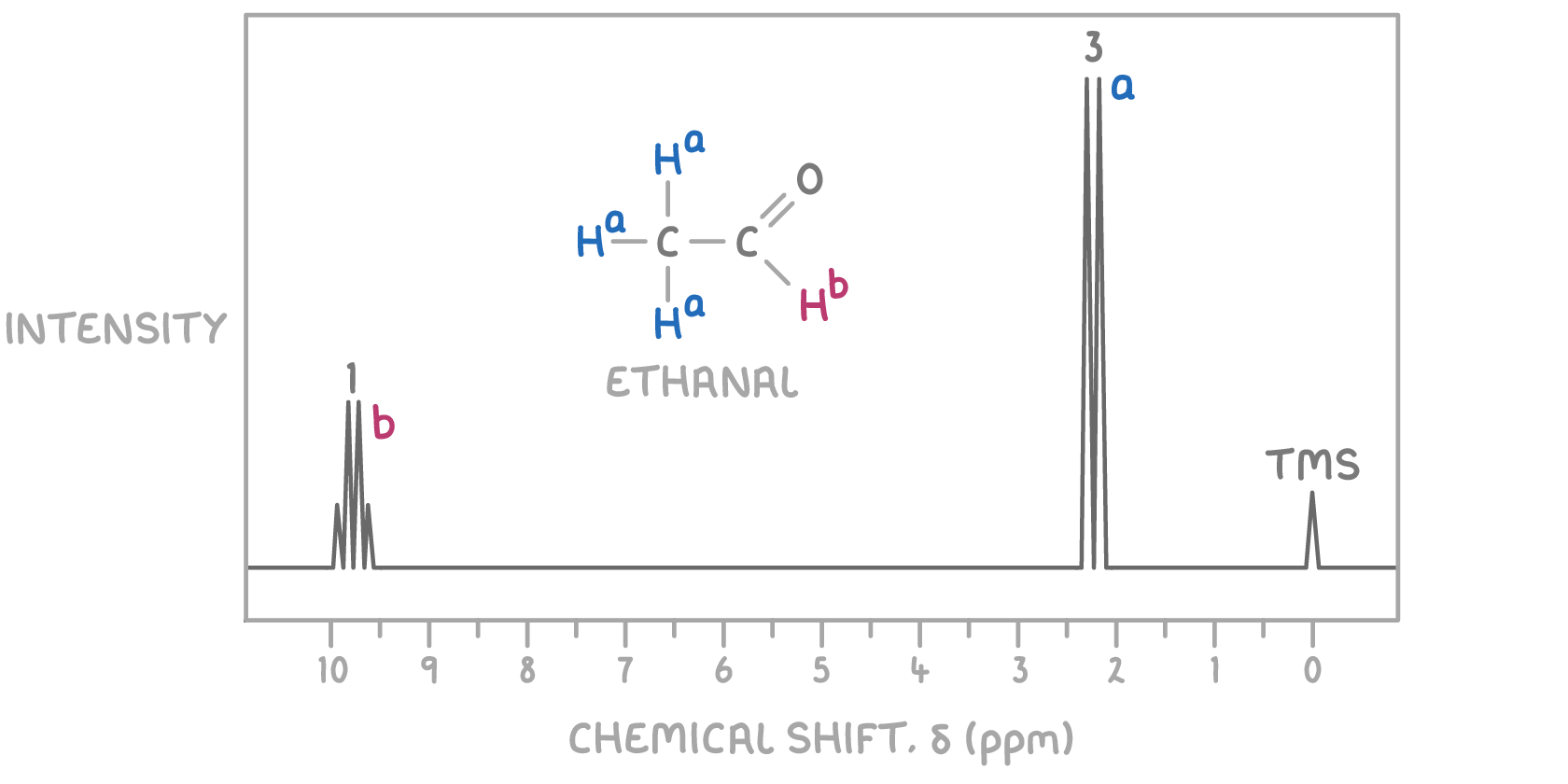
For example, in the 1H NMR spectrum of ethanal shown above:
- The peak at δ 2.2 ppm due to Ha is a doublet because there's one hydrogen on the adjacent carbon atom.
- The peak at δ 9.8 ppm due to Hb is a quartet because there are three hydrogens on the adjacent carbon atom.
Deuterated solvents are used in 1H NMR
To perform 1H NMR, the sample must be dissolved in a solvent. However, protons in common solvents like H2O would contribute unwanted peaks to the spectrum.
To overcome this issue, deuterated solvents are used, These solvents have their hydrogen atoms replaced with deuterium (2H), which produces signals outside the range observed in 1H NMR, ensuring only the sample's peaks are observed.
Common deuterated solvents include deuterium oxide (D2O) and deuterated chloroform (CDCl3).
Worked example 1 - Identifying the structure of an unknown molecule
Using the molecular formula C3H6O and the 1H NMR spectrum provided below, determine the structure of the unknown molecule.
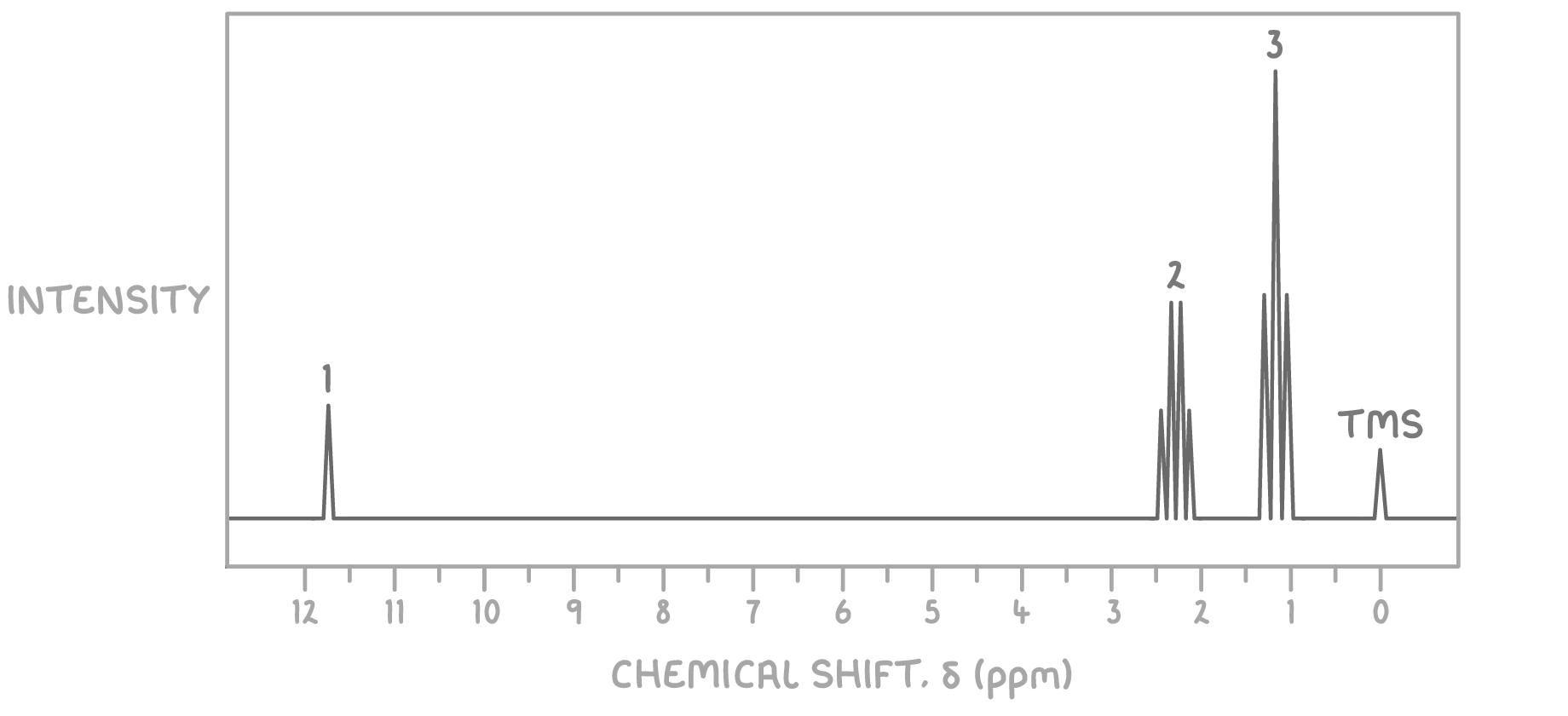
Step 1: Count signals
The presence of three distinct signals indicates three sets of magnetically non-equivalent hydrogen atoms in the molecule.
Step 2: Deduce environments from chemical shifts
- The signal at δ 1.2 ppm is a triplet, typically indicative of a methyl group (CH3) adjacent to a CH2 group.
- The signal at δ 2.4 ppm is a quartet, suggesting a CH2 group adjacent to a methyl group.
- The signal at δ 11.7 ppm is a singlet, characteristic of the acidic hydrogen (H) of a carboxylic acid.
Step 3: Consider the molecular formula
Given the molecular formula C3H6O2 and the signal patterns observed, the compound likely contains a carboxylic acid functional group, as indicated by the very downfield singlet at δ 11.7 ppm.
Step 4: Match environments to potential structures
Considering the NMR signal characteristics:
- The methyl group (CH3) giving rise to the triplet at δ 1.2 ppm suggests it is next to a CH2 group, which in turn shows as a quartet at δ 2.4 ppm due to splitting by the three hydrogen atoms of the methyl group.
- The singlet at δ 11.7 ppm clearly indicates the presence of the acidic hydrogen of the carboxylic acid group.
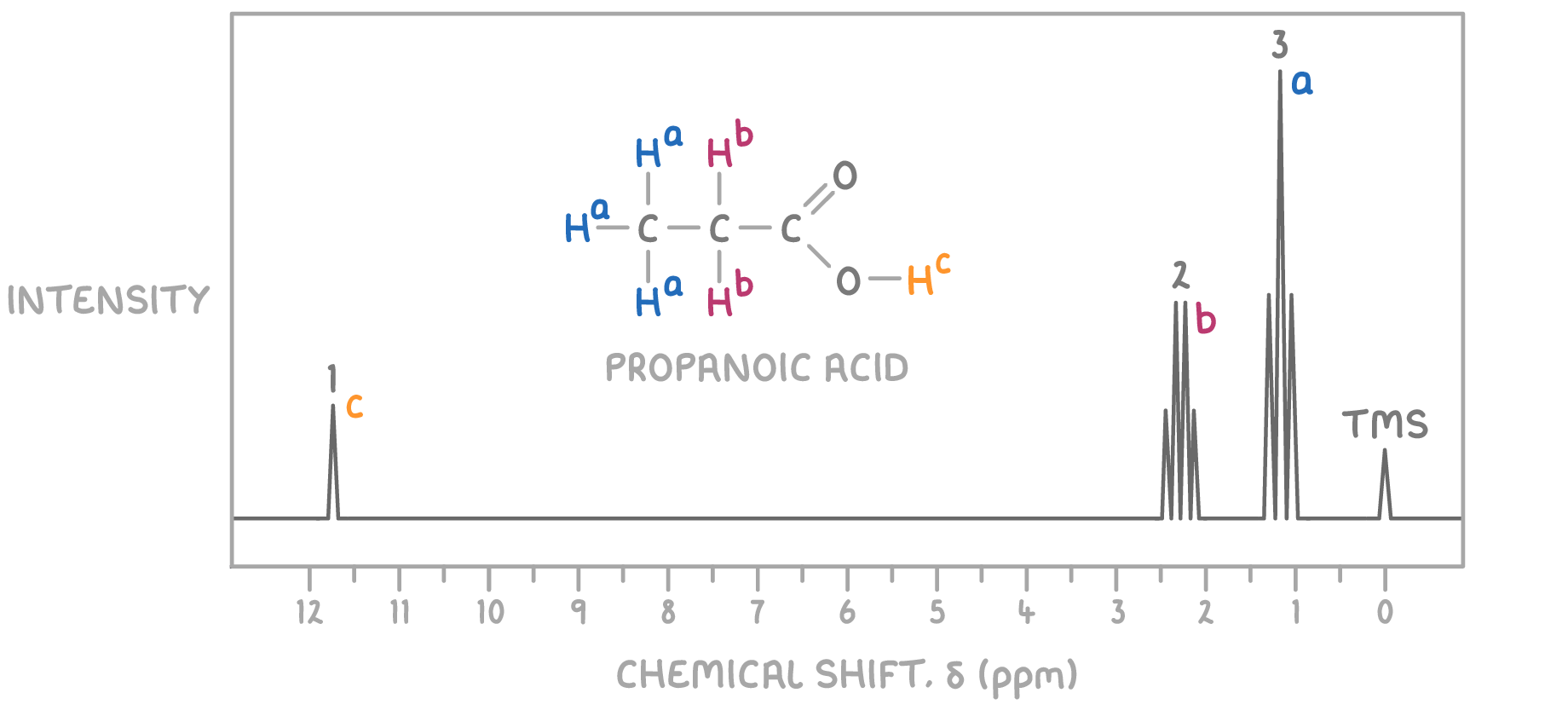
The structure that fits these criteria is propanoic acid, where the methyl group is responsible for the signal at δ 1.2 ppm, the CH2 group adjacent to the carboxylic acid gives rise to the signal at δ 2.4 ppm, and the carboxylic acid proton is responsible for the singlet at δ 11.7 ppm.
Therefore, the unknown molecule is identified as propanoic acid.
Worked example 2 - Identifying the structure of an unknown molecule
Using the molecular formula C4H8O2 and the 1H NMR spectrum provided below, determine the structure of the unknown molecule.
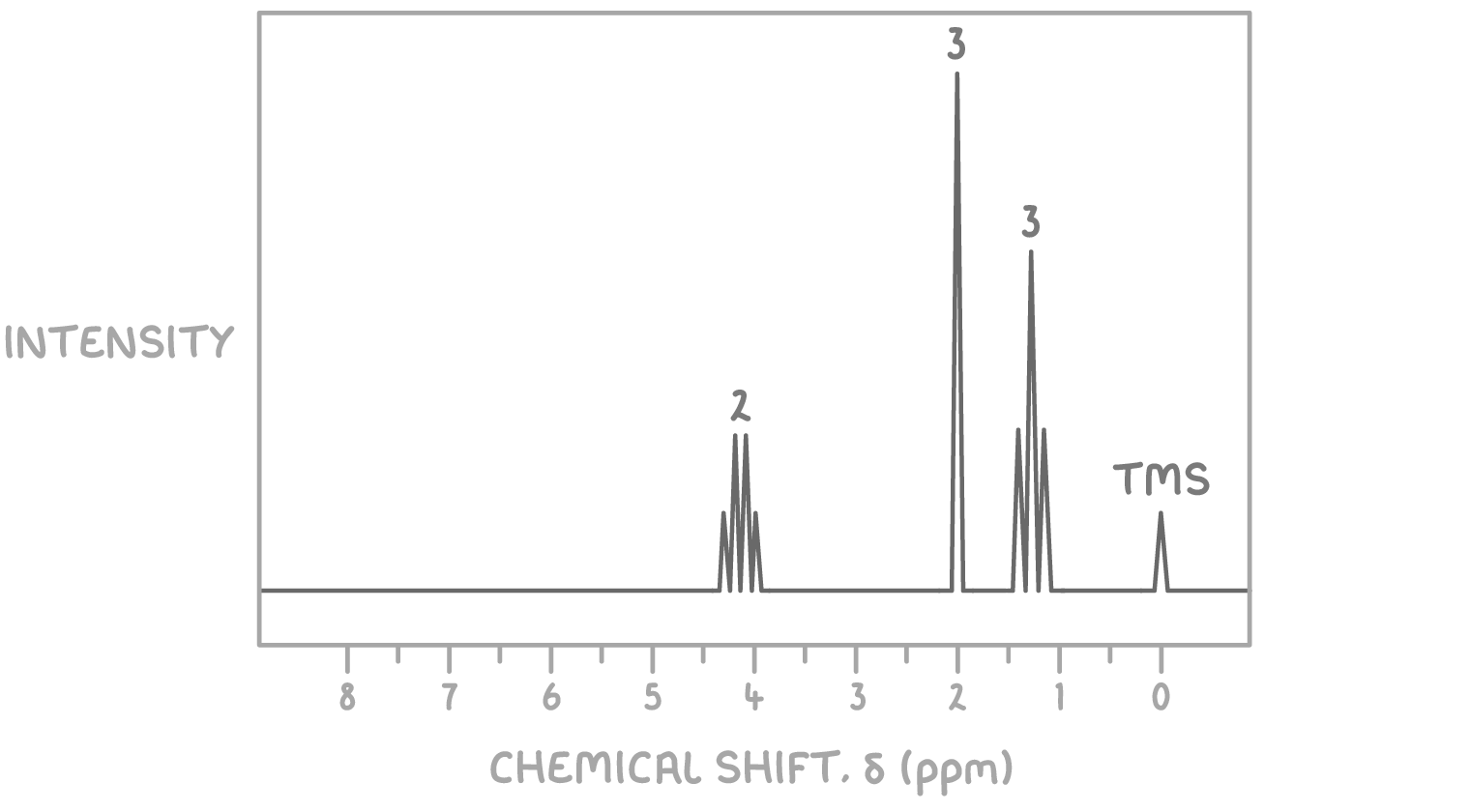
Step 1: Count signals
The presence of three distinct signals indicates three sets of magnetically non-equivalent hydrogen atoms in the molecule.
Step 2: Deduce environments from chemical shifts
- The signal at δ 1.3 ppm is a triplet, typically indicative of a methyl group (CH3) adjacent to a CH2 group.
- The signal at δ 2.0 ppm is a singlet, suggesting a hydrogen atom(s) on a carbon without neighbouring hydrogen atoms, commonly associated with the carbonyl group in esters or ketones.
- The signal at δ 4.1 ppm is a quartet, characteristic of a CH2 group adjacent to a methyl group, often observed in esters linked directly to oxygen.
Step 3: Consider the molecular formula
Given the molecular formula C4H8O2 and the signal patterns observed, the compound likely contains a functional group that accounts for the oxygen atoms, such as an ester, given the absence of NMR signals typical for hydroxyl (OH) or carboxylic acid (COOH) protons.
Step 4: Match environments to potential structures
Considering the NMR signal characteristics:
- The methyl group (CH3) giving rise to the triplet at δ 1.3 ppm suggests it is next to a CH2 group, which in turn shows as a quartet at δ 4.1 ppm due to splitting by the three hydrogen atoms of the methyl group. This CH2 group is likely attached to an oxygen atom, as indicated by its chemical shift.
- The singlet at δ 2.0 ppm can be attributed to the CH3 group attached to the carbonyl carbon, characteristic of esters.
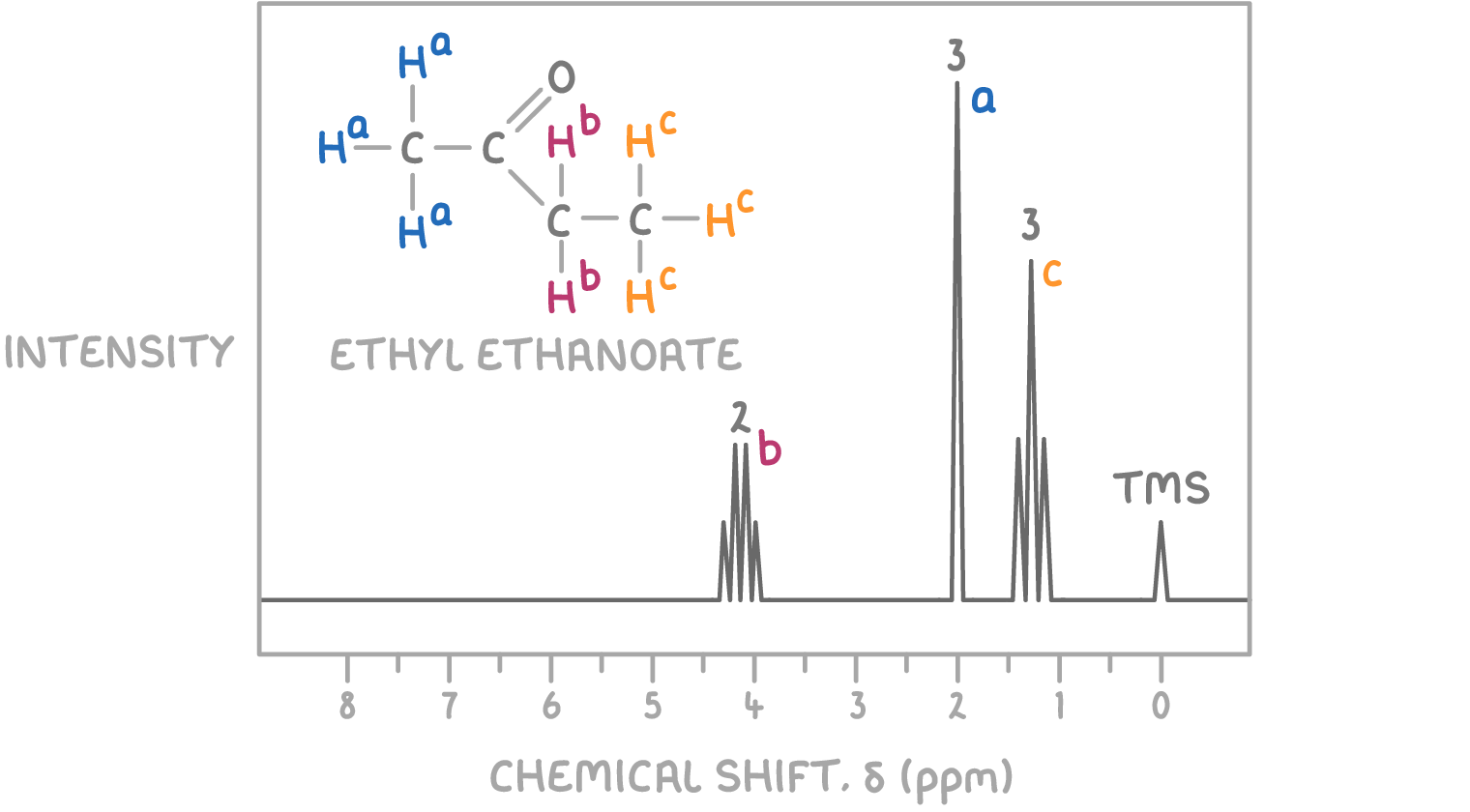
The structure that fits all these criteria is ethyl ethanoate, where the ethyl (CH3CH2-) part is responsible for the triplet and quartet, and the ethanoate (CH3COO-) part accounts for the singlet.
Therefore, the unknown molecule is identified as ethyl ethanoate.