Fundamental Particles, Mass Number and Atomic Number
This lesson covers:
- The subatomic particles that make up atoms
- How nuclear symbols represent the composition of atoms
- The difference between atoms and ions
Atoms are composed of protons, neutrons and electrons
All elements and compounds are made of atoms. These atoms consist of three fundamental subatomic particles: protons, neutrons, and electrons.

Protons and neutrons, collectively called nucleons, are located in the atom's nucleus. Protons have a positive charge, while neutrons are electrically neutral. The nucleus is extremely small and dense, containing the majority of an atom's mass.
Electrons, which have a negative charge, orbit the nucleus in shells or orbitals. Although electrons occupy a large region of space around the nucleus, most of an atom's volume is actually empty space.
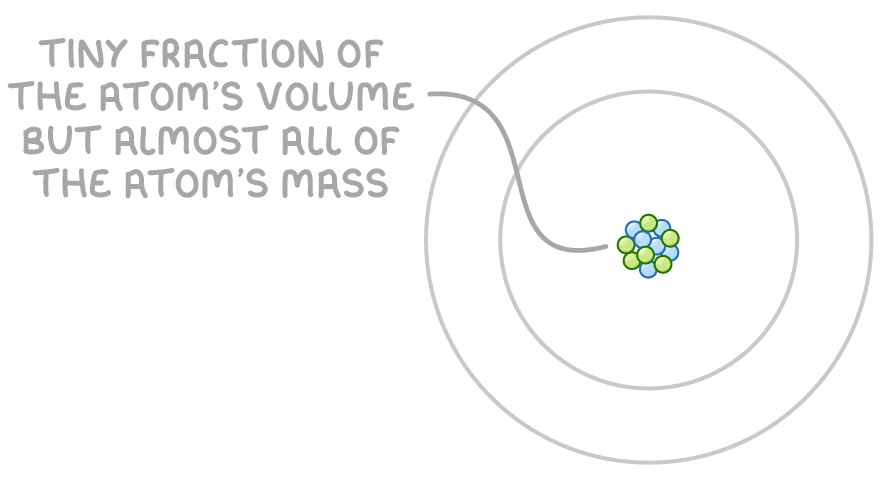
The properties of these subatomic particles are expressed using relative mass and relative charge:
| Subatomic particle | Relative mass | Relative charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 0.0005 | -1 |
Nuclear symbols represent atomic composition
Nuclear symbols provide a concise way to represent the composition of an atom. They indicate the number of protons, neutrons, and electrons in an atom using two key numbers:
- Mass number (A) - The total number of nucleons (protons and neutrons) in the nucleus.
- Atomic number (Z) - The number of protons in the nucleus, which uniquely identifies the element.
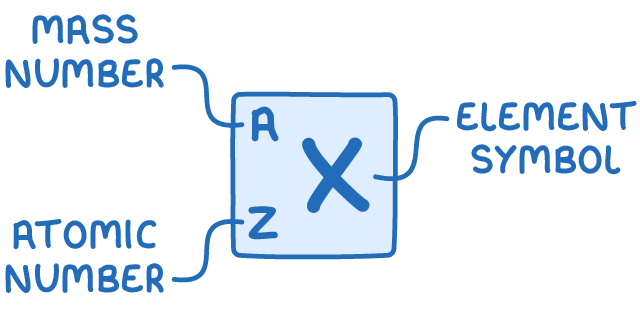
To determine the number of protons, electrons, and neutrons in a neutral atom:
- The number of protons is equal to the atomic number (Z).
- In a neutral atom, the number of electrons is equal to the number of protons.
- The number of neutrons is calculated by subtracting the atomic number (Z) from the mass number (A).
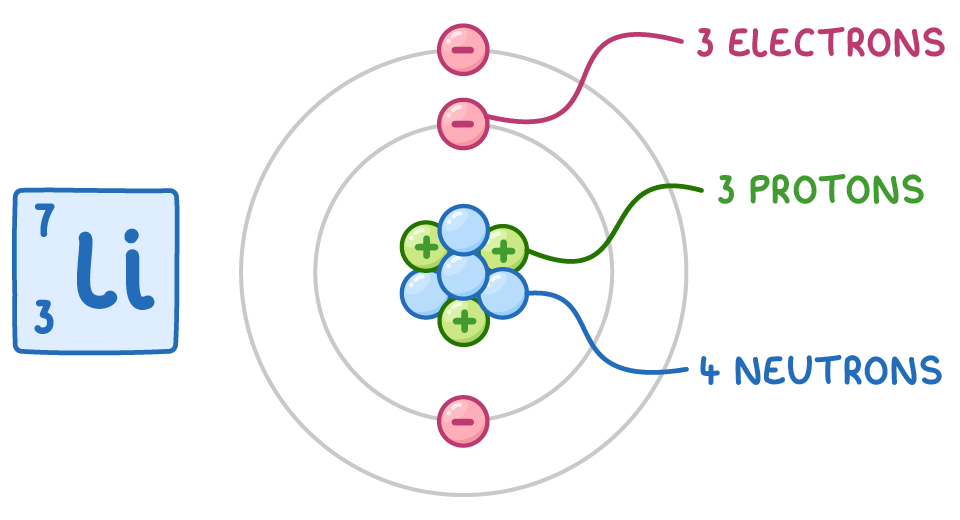
For example, let's consider the neutral atom 7Li:
- The atomic number (Z) is 3, so there are 3 protons.
- Since it is a neutral atom, the number of electrons is also 3.
- The mass number (A) is 7, so the number of neutrons is 7 - 3 = 4.
Therefore, a neutral 7Li atom has 3 protons, 3 electrons, and 4 neutrons.
The table below provides additional examples of how to use nuclear symbols to determine the number of protons, electrons, and neutrons in various neutral atoms:
| Ion | Atomic number | Mass number | Number of protons | Number of electrons | Number of neutrons |
|---|---|---|---|---|---|
| 19F- | 9 | 19 | 9 | 9 + 1 = 10 | 10 |
| 24Mg2+ | 12 | 24 | 12 | 12 - 2 = 10 | 12 |
| 32S2- | 16 | 32 | 16 | 16 + 2 = 18 | 16 |
Ions have unequal numbers of protons and electrons
Ions are atoms that have gained or lost electrons, resulting in an overall positive or negative charge.
- Negative ions (anions) have more electrons than protons. For example, a particle with 1 proton and 2 electrons would be a negative ion with an overall charge of -1.
- Positive ions (cations) have fewer electrons than protons. For example, a particle with 3 protons and 2 electrons would be a positive ion with an overall charge of +1.
To determine the number of protons, electrons, and neutrons in an ion:
- The number of protons is equal to the atomic number.
- The number of electrons is calculated by subtracting the ion's charge from the atomic number (for cations) or adding the ion's charge to the atomic number (for anions).
- The number of neutrons is calculated by subtracting the atomic number from the mass number.
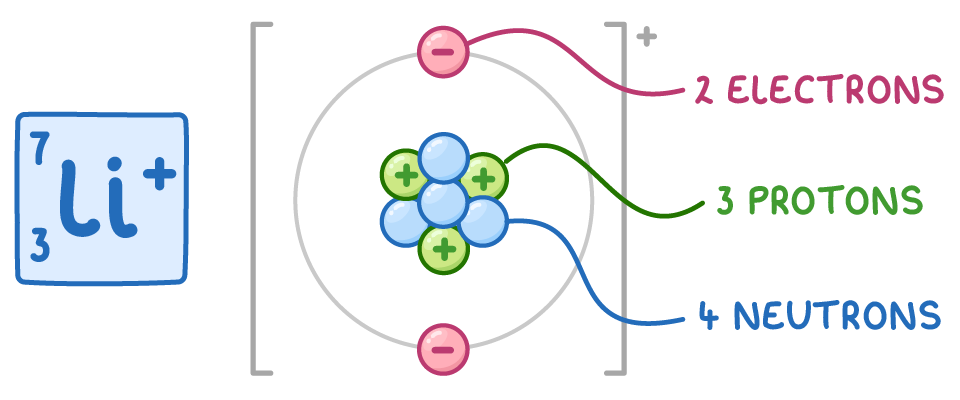
For example, in the 7Li+ ion:
- The atomic number is 3, so there are 3 protons.
- The ion has a + charge, so the number of electrons is 3 - 1 = 2.
- The mass number is 7, so the number of neutrons is 7 - 3 = 4.
Therefore, a 7Li+ ion has 3 protons, 2 electrons, and 4 neutrons.
The table below provides additional examples of how to calculate the numbers of protons, neutrons, and electrons for various positive and negative ions:
| Ion | Atomic number | Mass number | Number of protons | Number of electrons | Number of neutrons |
|---|---|---|---|---|---|
| 19F- | 9 | 19 | 9 | 9 + 1 = 10 | 10 |
| 24Mg2+ | 12 | 24 | 12 | 12 - 2 = 10 | 12 |
| 32S2- | 16 | 32 | 16 | 16 + 2 = 18 | 16 |