Enzymes
This lesson covers:
- What enzymes are and how they act as catalysts
- The lock and key model of enzyme specificity
- How inhibitors affect enzyme activity
- Enzyme inhibition in drug design
Enzymes are biological catalysts
Enzymes are proteins that speed up (catalyse) chemical reactions in living organisms:
- They reduce the activation energy needed for reactions to occur, making processes more efficient.
- Without enzymes, many reactions would occur too slowly to support life.
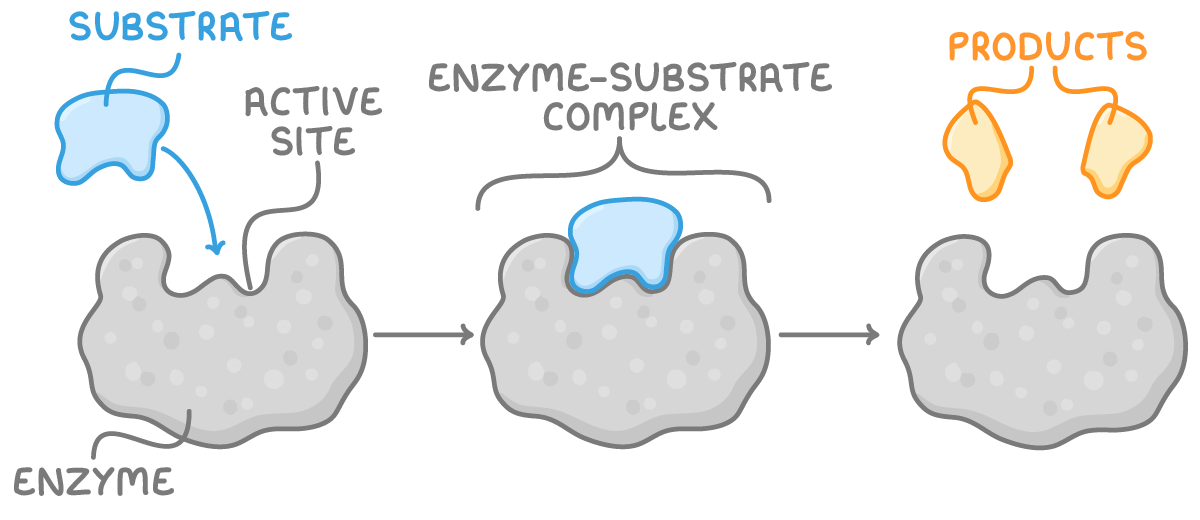
- Enzymes operate by attaching to specific molecules known as substrates, forming an enzyme-substrate complex. This interaction facilitates the chemical changes needed for the reaction.
- The specific region on an enzyme where substrates bind is called the active site. The precise fit of the substrate into the active site is crucial for the reaction to proceed.
- Once the reaction is complete, the resulting products are released from the active site, leaving the enzyme ready to interact with new substrates. This cycle allows enzymes to function effectively as catalysts.
Enzyme specificity
Enzymes are selective about the reactions they catalyse:
- The substrate must fit precisely into the active site, akin to a lock and key. This is known as the lock and key model.
- If the substrate's shape is not complementary to the active site, the reaction will not take place.
This specificity is due to the structure of enzymes:
- Enzymes are composed of amino acids, which include chiral centres.
- As a result, enzyme active sites are stereospecific, meaning they can only bind to one enantiomer of a chiral substrate.
Enzyme inhibition
Enzyme inhibitors are molecules that decrease the rate of enzyme-catalysed reactions.
There are primarily two types:
- Competitive inhibitors
- These molecules compete with the substrate for the active site.
- Their shape is similar to the substrate, allowing them to bind but not to react.
- Their effect can be reversed by increasing the concentration of the substrate.
- Non-competitive inhibitors
- These inhibitors attach to a different part of the enzyme, causing the active site to change shape.
- This distortion prevents the substrate from fitting correctly into the active site.
- Their effect cannot be reversed by simply increasing the substrate concentration.
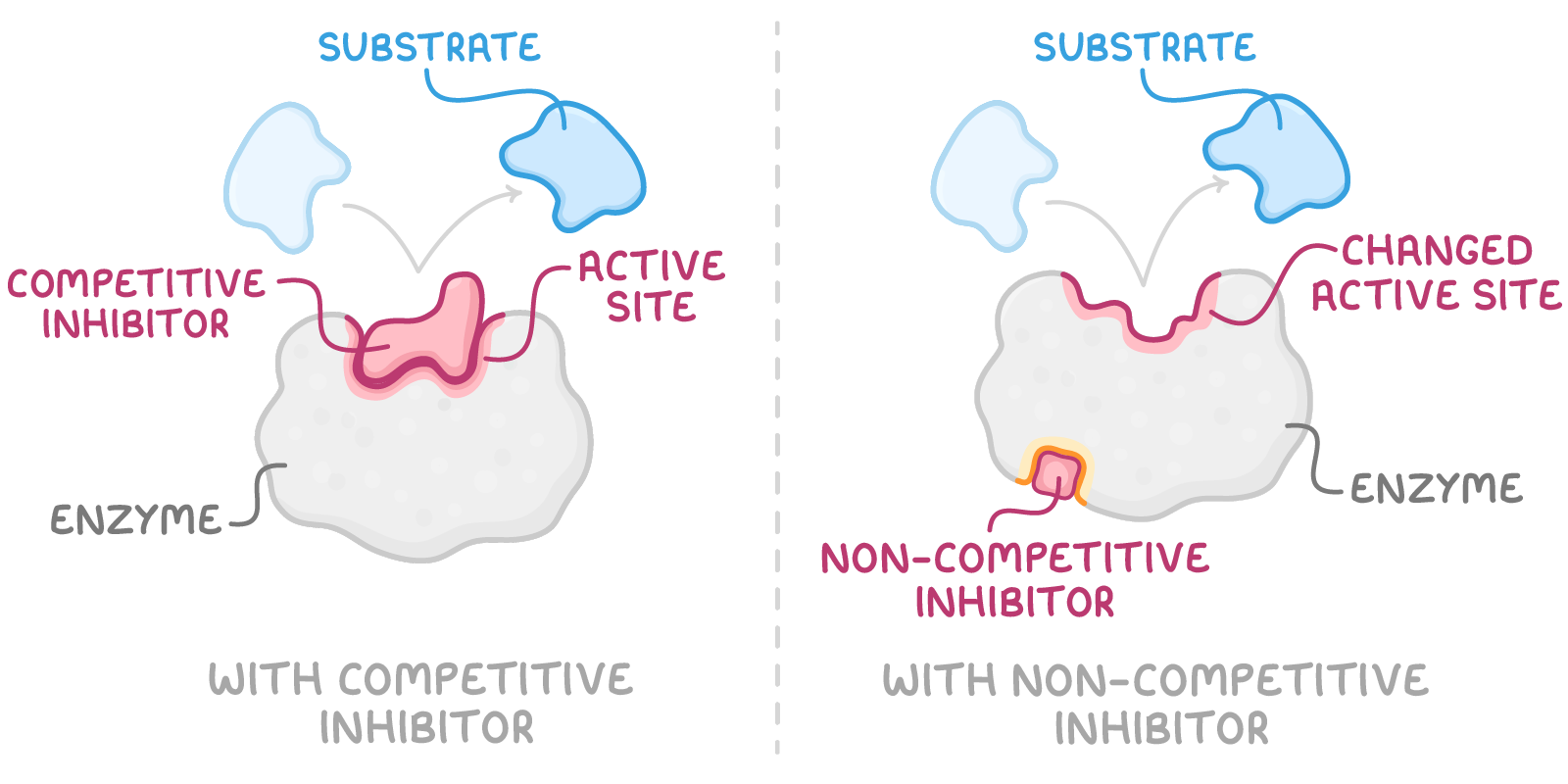
In both scenarios, the formation of the enzyme-substrate complex decreases over time, which in turn reduces the rate at which products are formed.
Many drugs work by inhibiting specific enzymes, for instance:
- Statins inhibit an enzyme necessary for cholesterol production, thereby lowering cholesterol levels.
- ACE inhibitors prevent the conversion of angiotensin, which helps manage high blood pressure.
- Antibiotics target bacterial enzymes essential for cell wall synthesis.
Applications in drug design
The study of enzymes plays a crucial role in the development of new drugs.
- Identifying new inhibitors:
- Virtual screening of drug libraries against models of the target enzyme's active site
- High-throughput screening to test numerous compounds for their ability to inhibit enzymes linked to diseases
- Optimising chirality:
- Many drug molecules are chiral.
- Computer models can predict which enantiomer will fit an enzyme's chiral active site, avoiding the need to synthesise and test ineffective forms.
These computational methods speed up the discovery of drugs that are safe, effective, and selective.