Chromatography
This lesson covers:
- What chromatography is and how it works
- Thin-layer chromatography (TLC)
- Column chromatography
- Gas chromatography (GC)
- Gas chromatography-mass spectrometry (GC-MS)
Chromatography separates mixtures
Chromatography is a group of laboratory techniques for separating the components of a mixture.
It involves two key elements:
- A mobile phase - A liquid or gas that carries the mixture through a system.
- A stationary phase - A solid or solid-supported liquid that does not move with the mobile phase.
The separation happens because of two main reasons:
- The mobile phase moves mixture components at varying speeds based on their solubility. Components that dissolve more easily move faster.
- The stationary phase holds onto the components differently through a process called adsorption. Components that are adsorbed more strongly move slower.
As a result, mixture components travel at different rates and separate into distinct layers, which can be individually analysed.
You'll learn about several types of chromatography in this lesson:
- Thin-layer chromatography (TLC)
- Column chromatography
- Gas chromatography (GC)
- Gas chromatography-mass spectrometry (GC-MS)
Thin-layer chromatography of mixtures
Thin-layer chromatography (TLC) is a simple and inexpensive form of chromatography used to analyse mixtures.
How TLC works:
- A stationary phase, typically silica gel or alumina, is spread thinly on a support material like glass or metal.
- A small amount of the mixture to be analysed is applied close to the plate's bottom edge.
- The plate is then placed upright in a sealed container with a small amount of solvent or solvent mixture at the bottom.
- The solvent moves up the plate by capillary action, carrying the mixture components at different rates based on their solubility in the solvent and their affinity for the stationary phase.
- Once the solvent has nearly reached the top, the plate is removed, and the locations of the separated components are marked.
Using Rf values to identify substances:
The retention factor (Rf) value is the ratio of the distance travelled by a component (spot) from the baseline to the distance travelled by the solvent. It is calculated using the following formula:
Rf =distance travelled by soventdistance travelled by spot
These distances are represented on the chromatogram below.
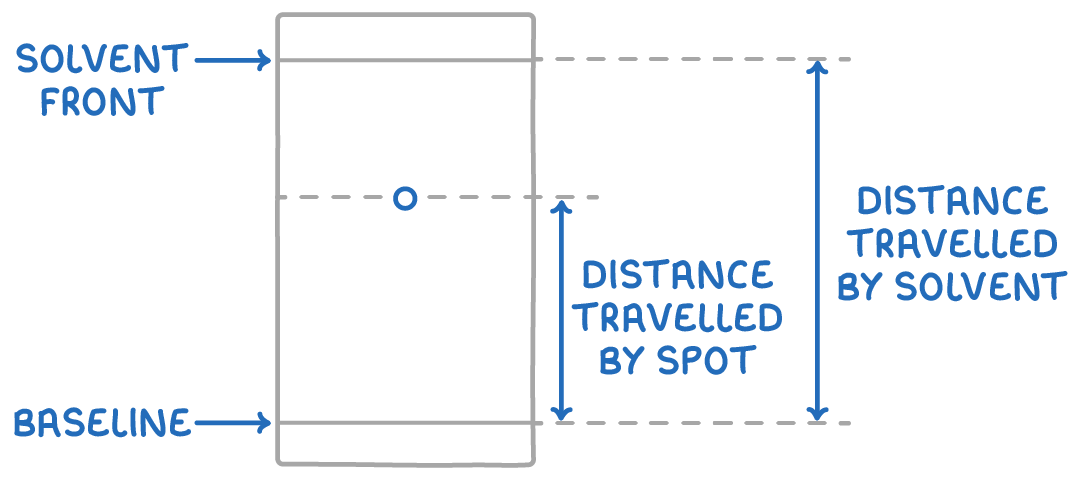
Rf values range from 0 to 1 and can help identify compounds based on their movement under specific conditions. Comparing the Rf values of unknown substances to those of known compounds, under the same conditions, can reveal their identities.
Column chromatography to purify solutions
Column chromatography is a technique for purifying individual components from mixtures. It uses a glass column filled with a solid adsorbent as the stationary phase.
How column chromatography works:
- The mixture is placed at the top of the column filled with the stationary phase.
- A solvent is passed through the column, carrying the mixture components at different rates.
- Components separate based on their solubility in the solvent and their adsorption to the stationary phase.
- The separated components exit the column and are collected for analysis.
Gas chromatography of volatile liquids
Gas chromatography (GC) separates volatile liquid mixtures into individual substances.
How gas chromatography works:
- A liquid sample is vaporised in a heated chamber.
- An inert carrier gas then transports the vaporized sample through a chromatographic column containing the stationary phase, consisting of either an immobilised solid or a solid coated with a non-polar high boiling liquid.
- The column has a stationary phase that interacts differently with each component of the sample.
- Components are separated based on how they partition between the mobile gas phase and the stationary phase.
- As they exit the column, a detector records the separation, producing a chromatogram with distinct peaks for each component.
Using retention times to identify substances
Gas chromatograms display peaks that represent different substances in a mixture. The position of each peak is determined by its retention time, which is the time taken for the corresponding substance to travel through the chromatographic column.
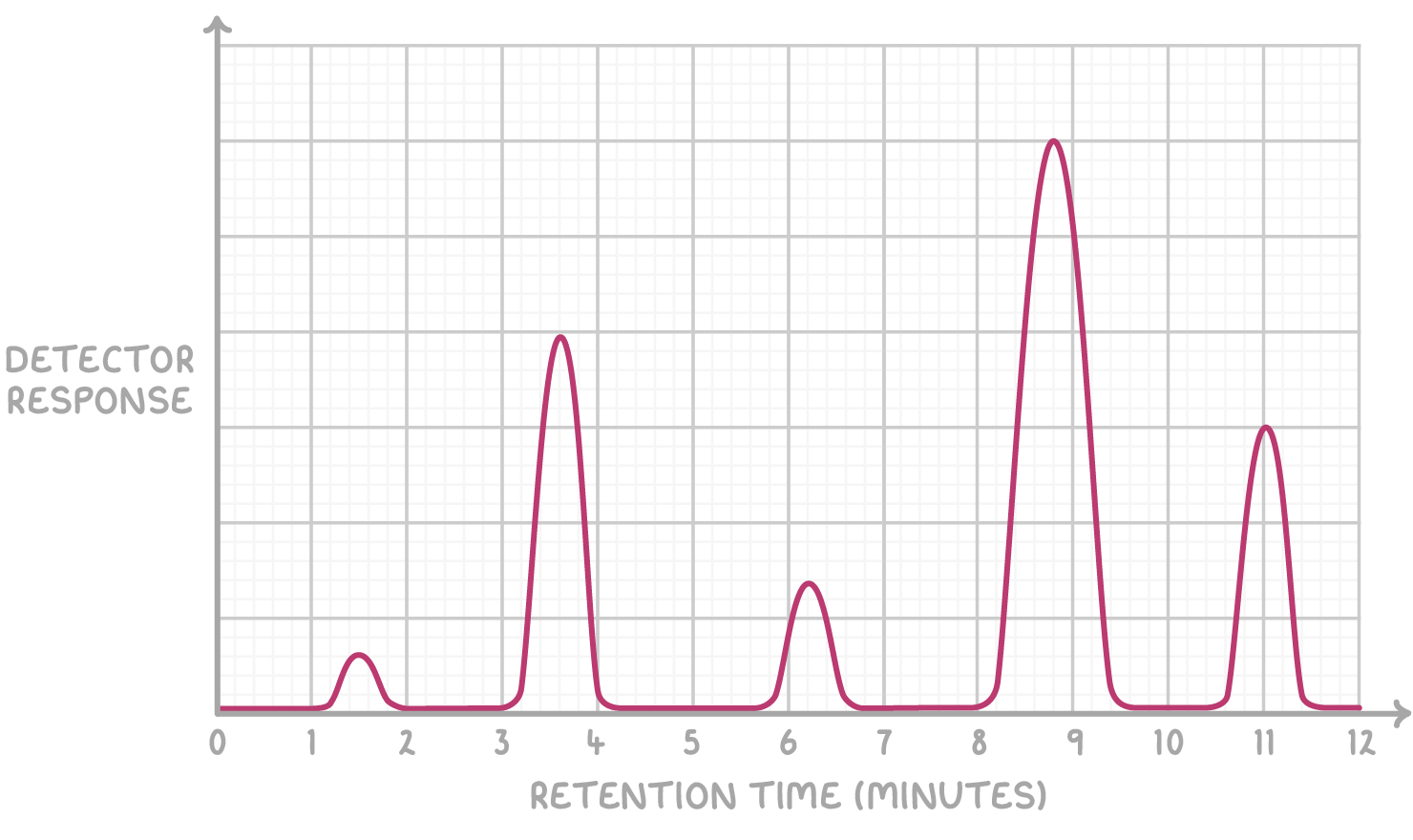
To identify the substances in the mixture, compare the retention times of the peaks with those of known standards.
Gas chromatography-mass spectrometry (GC-MS)
GC-MS combines the separation capabilities of gas chromatography with the identification power of mass spectrometry, making it a highly effective method for analysing complex mixtures.
How GC-MS works:
- The mixture is first separated by gas chromatography.
- Each component is then introduced into a mass spectrometer, where it is ionised.
- A distinct mass spectrum for each component is generated.
- Computerised matching to spectral libraries then identifies each substance based on their characteristic fragmentation patterns.
GC-MS provides detailed information, enabling the precise identification and quantification of substances within a mixture in a single, automated process.