Alcohols
This lesson covers:
- What 'alcohols' are
- The properties of alcohols
- The uses of alcohols

Alcohols are a homologous , with a functional group of .
|
What is the general formula for alcohols?
CnH2nOH
CnH2n+1OH
CnH2n+2OH
|
An alcohol has six carbon atoms and one OH group.
What is its chemical formula?
C6H11OH
C6H14OH
C6H13OH
C6H12OH
|
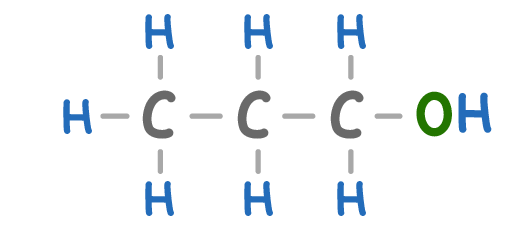
Alcohols have a similar name to alkanes except the 'e' at the end is replaced by an 'ol'.
What is the name of the molecule above?
|
What is the displayed formula of butanol?
When you're ready to check your answer, click 'Continue'.
|
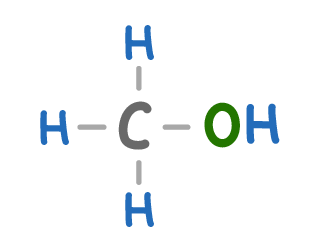
What is the molecule above called?
Methane
Methene
Methanol
Methanoic acid
|
What are the properties of alcohols?
(Select all that apply)
They are soluble
They are flammable
They are unsaturated
They can be oxidised to carboxylic acids
|
All alcohols can undergo combustion reactions to form CO2 and H2O.
Balance this combustion reaction:
C4H9OH + O2 ➔ CO2 + H2O
|
solidify / dissolve / 5 / 7 / 9
Alcohols can in water.
Alcohols are not acidic or alkaline, therefore their pH is .
|
What are some uses of alcohols?
(Select all that apply)
As a solvent in industry
As a fertiliser
As a fuel
|