Formation of Ions
This lesson covers:
- How ions are made
- Why some elements are more likely to form ions than others
- How to write equations for the ionisation of an atom
positive / negative / bigger / smaller / shell
Atoms need a full outer of electrons in order to be stable. One way they can achieve this is by gaining or losing electrons.
Those that gain electrons become ions with a charge, whilst those that lose electrons become ions with a charge.
|
Magnesium loses electrons to become an ion. Complete the equation below:
➔ Mg2+ + e-
|
Choose the correct equation for the ionisation of a bromine atom:
Br + 2e- ➔ Br2-
Br + e- ➔ Br-
Br ➔ 2e- + Br-
|
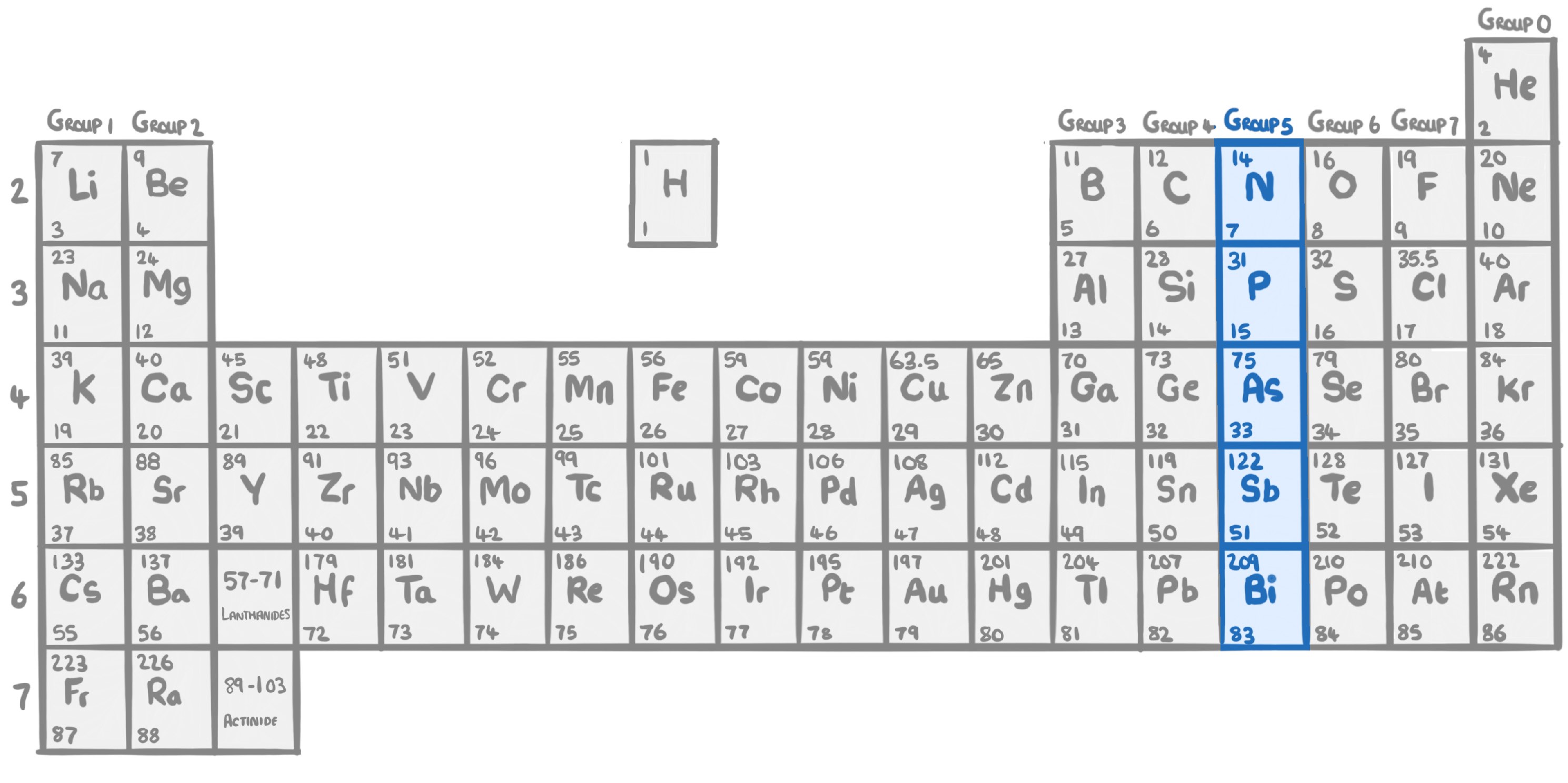
Atoms in group 5 have electrons in their outer shell. This means they need to gain electrons to be stable, forming an ion with a charge of .
|
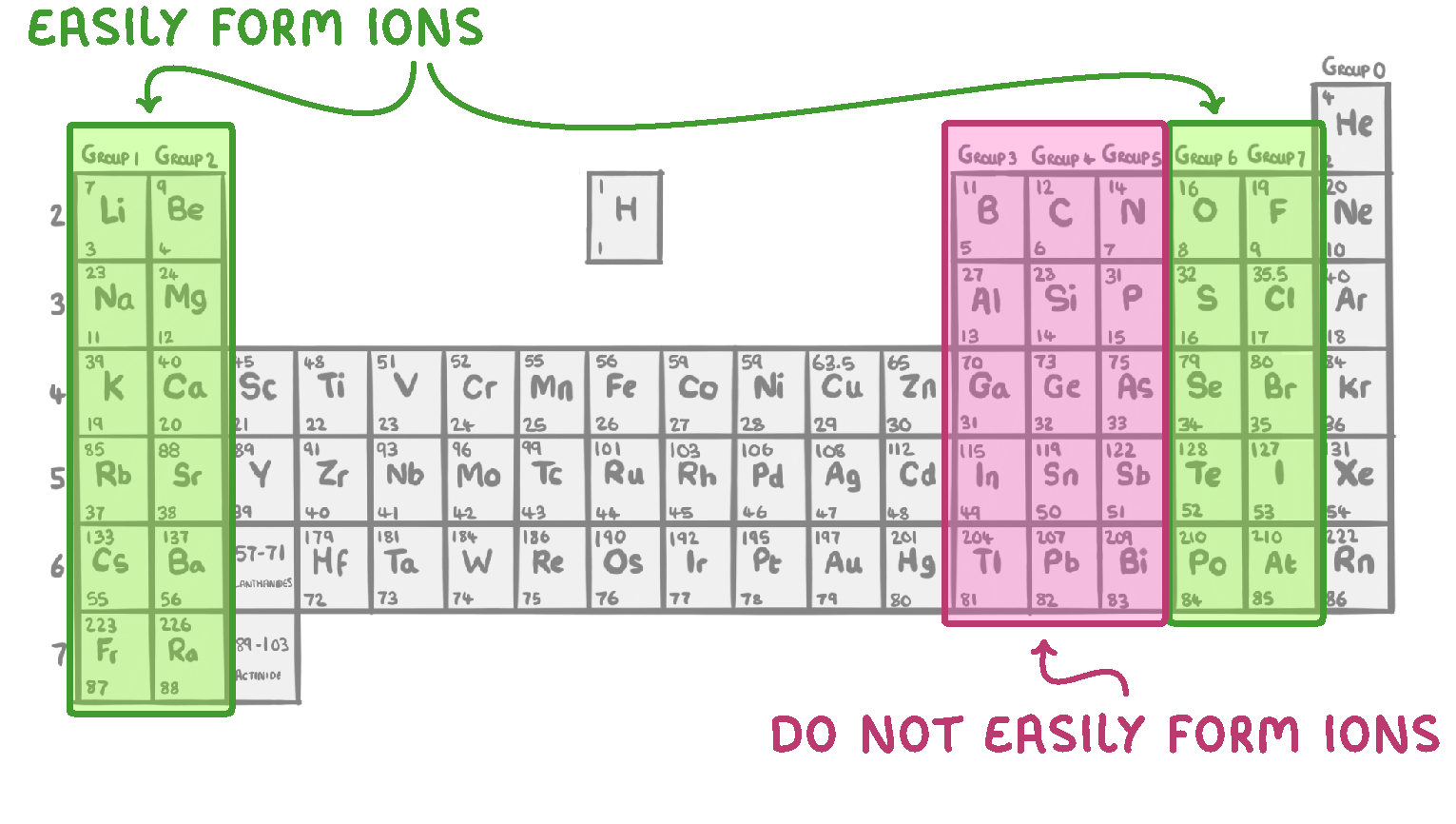
The transfer of electrons requires a lot of energy.
Groups 1, 2, 6 and 7 form ions very easily. This is because they only need to gain or lose a small number of electrons, and so less energy is required.
Those in groups 3, 4 and 5 do not form ions very easily, because they need to gain or lose a larger number of electrons, and therefore more energy is required.
more / less / time / energy
Atoms which need to gain or lose only 1-2 electrons are likely to become ions, compared to those which need to gain or lose 3-4.
This is because gaining or losing electrons requires a lot of .
|