Atoms
This lesson covers:
- The structure of atoms
- The properties of protons, neutrons, and electrons
- How to read the periodic table

What is the charge on a proton?
Positive
Negative
Neutral
|
Which of the following make up the nucleus of an atom?
(Select all that apply)
Neutron
Electron
Proton
|

Which particle determines what element an atom is?
Protons
Neutrons
Electrons
|
Which particles within an atom have a relative mass of 1?
(Select all that apply)
Neutrons
Protons
Electrons
|
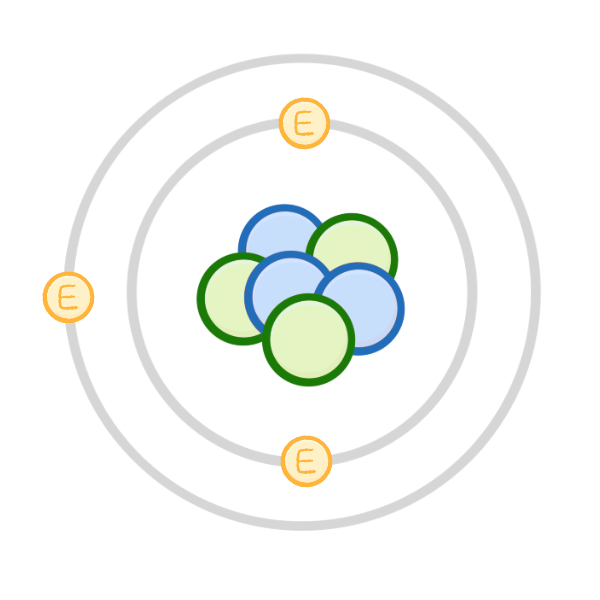
Which sub-atomic particle does NOT have a charge?
|
What do we call an atom that has a positive or negative charge?
An element
A positron
A molecule
An ion
|

What is the charge on an electron?
Positive (+1)
Negative (-1)
Neutral
|
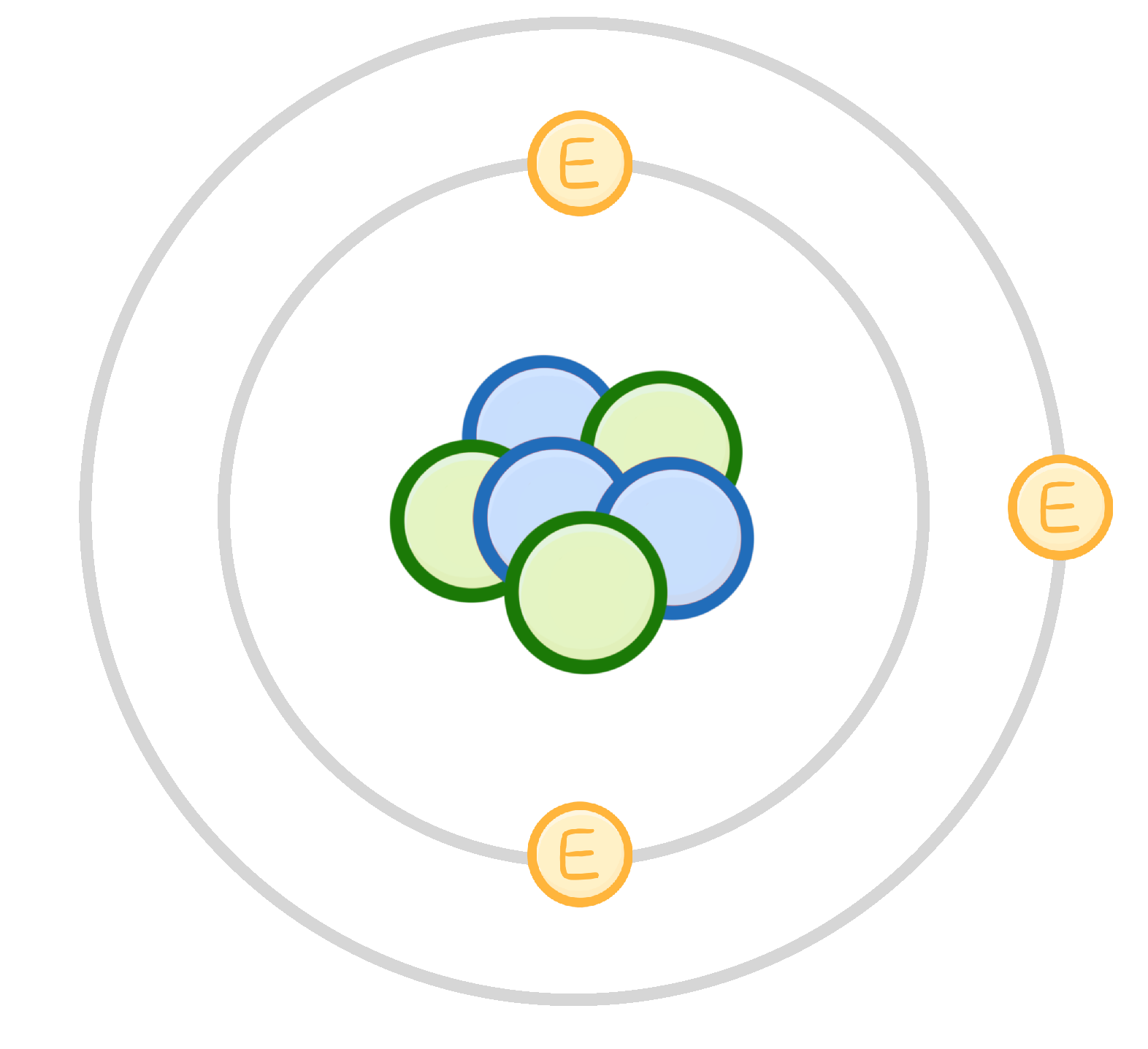
Electrons orbit the nucleus in energy s.
|
If an atom has 6 protons and 5 electrons, what charge will it have overall?
-2
-1
0
+1
+2
|