Tests for Cations
This lesson covers:
- What 'cations' are
- How the 'flame test' for cations works
- What happens when cations react with sodium hydroxide
The term 'cation' just means a positively charged ion. For example Mg2+, Na+, and Al3+ ions are all cations.
Nearly all cations are metals ions, as metals form positive ions. The only exception you're likely to come across is the ammonium ion, NH4+, which is a non-metal cation.
In this lesson we'll be looking at how to test for these metals ions.
Which of the following is a cation?
OH-
H2O
Ca2+
|
There are two groups of tests for cations that you need to know about:
- The flame tests
- The metal hydroxide tests
Flame tests One way we can test for metal ions is to see what colour flames they produce when they burn. Different metal ions produce different colours when burning, and so we can use the colour of the flame to help identify the type of metal. |
The steps of the procedure
|
2Dip the wire loop into the compound you want to test. |
3Hold the wire loop in the clear blue part of the Bunsen burner flame (this is the hottest part). |
4See what colour the flame turns as the compound burns. |
In preparation for the flame test, which two of the following are used to clean the platinum wire loop?
Hydrochloric acid
Antiseptic wipes
Bunsen burner flame
Soapy water
|
When performing the flame test, in which part of the flame should you place the wire loop with the metal sample?
Yellow part of the flame
Orange part of the flame
Blue part of the flame
|
The colour of each metal ion's flame |
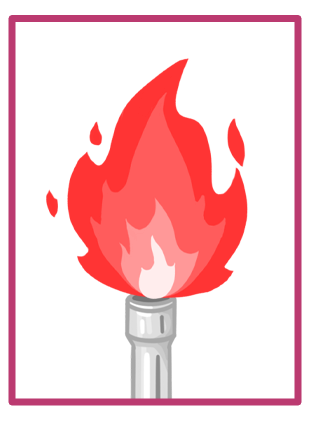 1Lithium ions (Li+) burn with a crimson flame. |
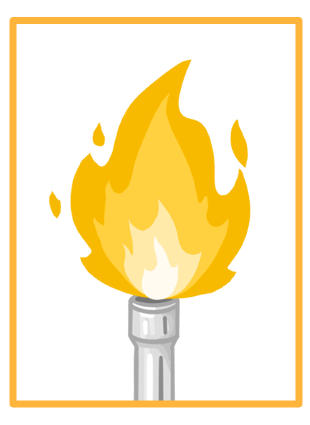 2Sodium ions (Na+) burn with a yellow flame. |
 3Potassium ions (K+) burn with a lilac flame. |
 4Calcium ions (Ca2+) burn with an orange-red flame. |
5Copper ions (Cu2+) burn with a green flame.  |
In the flame test for cations, which metal burns with a lilac flame?
Lithium ions
Potassium ions
Calcium ions
|
In the flame test for cations, what colour flame do calcium ions give?
Orange-red
Yellow
Green
|
In the flame test for cations, which metal burns with a crimson flame?
Lithium ions
Sodium ions
Copper ions
|
In the flame test for cations, what colour flame do sodium ions give?
Yellow
Green
Orange-red
|
In the flame test for cations, what colour flame do copper ions give?
Green
Orange-red
Yellow
|
What if there are multiple metals in a sample?

A limitation of the flame test is that if you have 2 or more different metals in your sample, then the colours of the flames will mix together, and you probably won't be able to tell which metals you have.
The metal hydroxide test |
Another test we can do for metal ions is react them with a solution of sodium hydroxide (NaOH), and see what colour the solution turns. |
This is possible because some metal ions form coloured precipitates when they react with hydroxide ions, and these coloured precipitates then determine the colour of the solution. |
For example, when copper(II) reacts with hydroxide ions, it forms a blue precipitate of copper hydroxide. This means the entire solution turns a blue colour. Cu2+(aq) + 2OH-(aq) ➔ Cu(OH)2 (s) |
You also need know how sodium hydroxide reacts with calcium ions, iron(II) ions, iron(III) ions, magnesium ions, and aluminium ions. |
Calcium (formula: Ca2+) forms a white precipitate. Ca2+(aq) + 2OH-(aq) ➔ Ca(OH)2 (s) |
Iron(II) (formula: Fe2+) forms a green precipitate. Fe2+(aq) + 2OH-(aq) ➔ Fe(OH)2 (s) |
Iron(III) (formula: Fe3+) forms a brown precipitate. Fe3+(aq) + 3OH-(aq) ➔ Fe(OH)3 (s) |
Magnesium (formula: Mg2+) forms a white precipitate. Mg2+(aq) + 2OH-(aq) ➔ Mg(OH)2(s) |
Aluminium (formula: Al3+) forms a precipitate that is white at first, but if there is excess NaOH, it then redissolves to form a colourless solution. Al3+(aq) + 3OH-(aq) ➔ Al(OH)3 (s) |
In the sodium hydroxide test for cations, which ion gives a brown precipitate?
Ca2+
Fe2+
Mg2+
Fe3+
|
In the sodium hydroxide test for cations, which two ions give a white precipitate?
Ca2+
Fe2+
Fe3+
Mg2+
|
In the sodium hydroxide test for cations, which colour precipitate do Cu2+ ions give?
Green
Blue
Brown
Orange
|
In the sodium hydroxide test for cations, which ion gives a white precipitate at first, but then redissolves to give a colourless solution when excess sodium hydroxide is added?
Al3+
Fe2+
Ca2+
Cu2+
|