The History of The Atom
This lesson covers:
- The theories and experiments that led to our current understanding of the atom
For this topic, you don't need to remember all of the dates, but it's helpful to have an idea of the timeline.
When Democritus first conceived of atomic theory, around 500 BC, how did he describe atoms?
(Select all that apply)
Tiny living creatures
Separated from each other by empty space
Small spheres
The smallest possible unit of matter
|
rocks / cubes / spheres / atoms / elements
In the 1800's, John Dalton described atoms as solid , and suggested that different types of spheres make up the different .
|
In 1897 J. J. Thomson theorised that an atom consisted of a ball of positive charge, with negative electrons mixed throughout it. What do we call this model?
Plum pudding model
Nuclear model
Atomic model
Quantum Model
|
How Rutherford developed the nuclear model
beta / deflected / positive / alpha / negative
- In Rutherford's experiments, particles were fired at a thin sheet of gold foil.
- Most particles passed through, but some were off course.
- This caused him to hypothesise that there was a dense region of charge at the centre of the atom that repelled the alpha particles.
- As a result he developed the nuclear model of the atom, in which there was a central positive nucleus, surround by electrons.
|
One issue with Rutherford's nuclear model was that the atom should collapse as the negative electrons would be attracted to the positive nucleus, causing them to rush inwards.
In response to this, in 1913 Bohr suggested that electrons ___________________, which prevents the atom from collapsing.
orbit the nucleus in shells
have magnetic repulsion
are actually positively charged
|
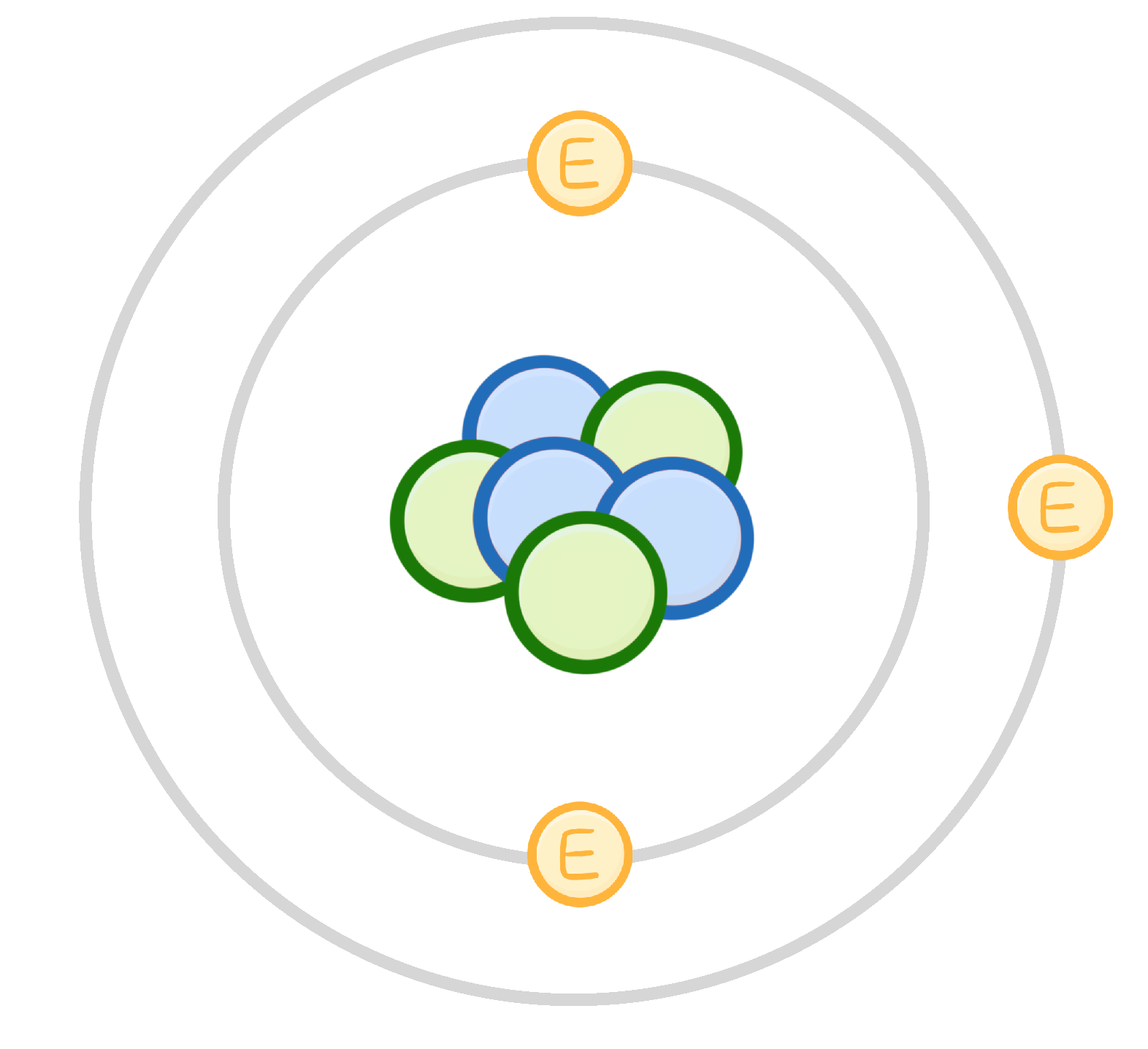
In the 20th century, Chadwick discovered neutral particles in the atomic nucleus. What are these particles called?
Electrons
Protons
Neutrons
|