Cracking & Alkenes
This lesson covers:
- How longer hydrocarbons can be broken down into shorter hydrocarbons through 'cracking'
- The two types of cracking reactions: 'catalytic cracking' and 'steam cracking'
- How to complete a balanced chemical equation for cracking
- An introduction to 'alkenes'
- The 'bromine water test' to distinguish between alkenes and alkanes
higher / lower / more / less
Hydrocarbons with different length chains have different properties, so can be used for different things.
Shorter chain alkanes have melting and boiling points, so are flammable, and volatile.
|
smaller / larger
Cracking is the process in which chain hydrocarbons are split into , more useful hydrocarbons.
|
What type of reaction is cracking?
Redox reaction
Combustion reaction
Acid base reaction
Thermal decomposition reaction
|
Catalytic cracking
- First, some long chain alkanes are until they vaporise into a
- Then they're passed over a hot, powdered oxide catalyst
- This breaks the long chain alkanes into a chain alkane and an
|
How is steam cracking different to catalytic cracking?
low / high / steam / catalyst / rate
Steam cracking is different because there is no involved. Instead the vaporised long chain alkane is mixed with at very temperatures.
|
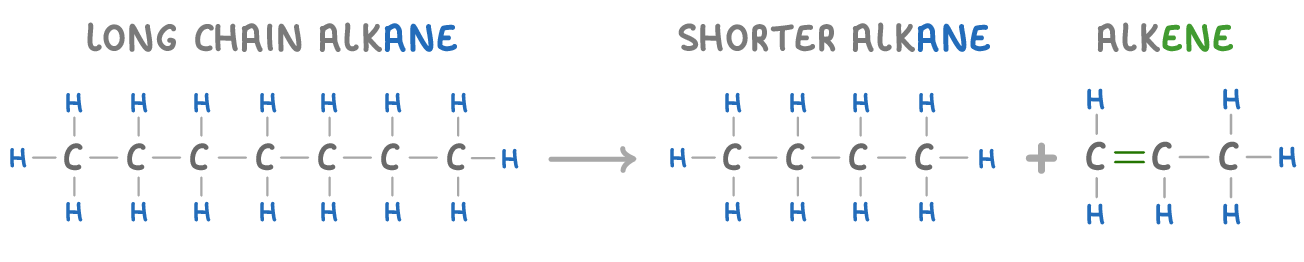
carbon / hydrogen / alkane
When a long chain is cracked, there aren't enough atoms to make two alkanes. Hence, cracking will always produce one alkane and one alkene. (Alkenes are hydrocarbons with a double bond between two carbon atoms).
|
True or false? In cracking, the number of carbon and hydrogen atoms in the reactants and products are always balanced.
True
False
|
? ➔ C3H6 + C5H12
An unknown alkane was cracked to produce propene (C3H6) and pentane (C5H12).
What is the unknown alkane?
Nonane C9H20
Hexane C6H12
Octane C8H18
Heptane C7H16
|
C9H20 ➔ C2H4 + C?H?
Nonane (C9H20) is cracked to produce ethene (C2H4) and one other product.
What is the other product?
Heptene C7H14
Heptane C7H16
Hexane C6H14
Hexene C6H12
|
Introduction to alkenes
saturated / unsaturated / homologous / carbon / hydrogen
- Alkenes are similar to alkanes. Alkenes are also hydrocarbons and also an example of a series.
- The difference is that alkenes have a double bond between two atoms, whereas alkanes only have single bonds.
- Another way to express the presence of the double bond between two carbon atoms is to say that alkenes are ''.
|
The double bond in alkenes makes alkenes ________ reactive than alkanes.
less
more
|
Bromine test for alkenes
chlorine / bromine / iodine / orange / blue / colourless
The test to distinguish between alkanes and alkenes is the water test.
Bromine water (just bromine dissolved in water) by itself is an colour. But when it's mixed with alkenes, all of the bromine will react, and so the solution loses its orange colour, and turns .
This doesn't happen when bromine water is mixed with alkanes, because alkanes are not reactive enough to react with bromine water. So when mixed with an alkane, the solution will stay orange.
|