Fractional Distillation
This lesson covers:
- What 'crude oil' is
- How crude oil is formed
- How to separate crude oil into its separate components, using fractional distillation
reaction / hydrocarbons / mixture
Crude oil is a of many different compounds. Most of the compounds are .
|

Is crude oil is a finite or renewable resource?
Finite
Renewable
|
How crude oil was made:
dinosaurs / plankton / pressure / buried / thousands / millions
- Crude oil is formed from the remains of dead plants and animals, particularly .
- These organic remains were covered by mud and sand, and in the earth.
- Over of years, these organic remains were compressed under a lot of heat and pressure.
- The heat and chemically changed the organic remains into crude oil.
|
Crude oil is a fossil fuel. What else is an example of a fossil fuel?
(Select all that apply)
Wood
Geothermal energy
Coal
Natural gas
|
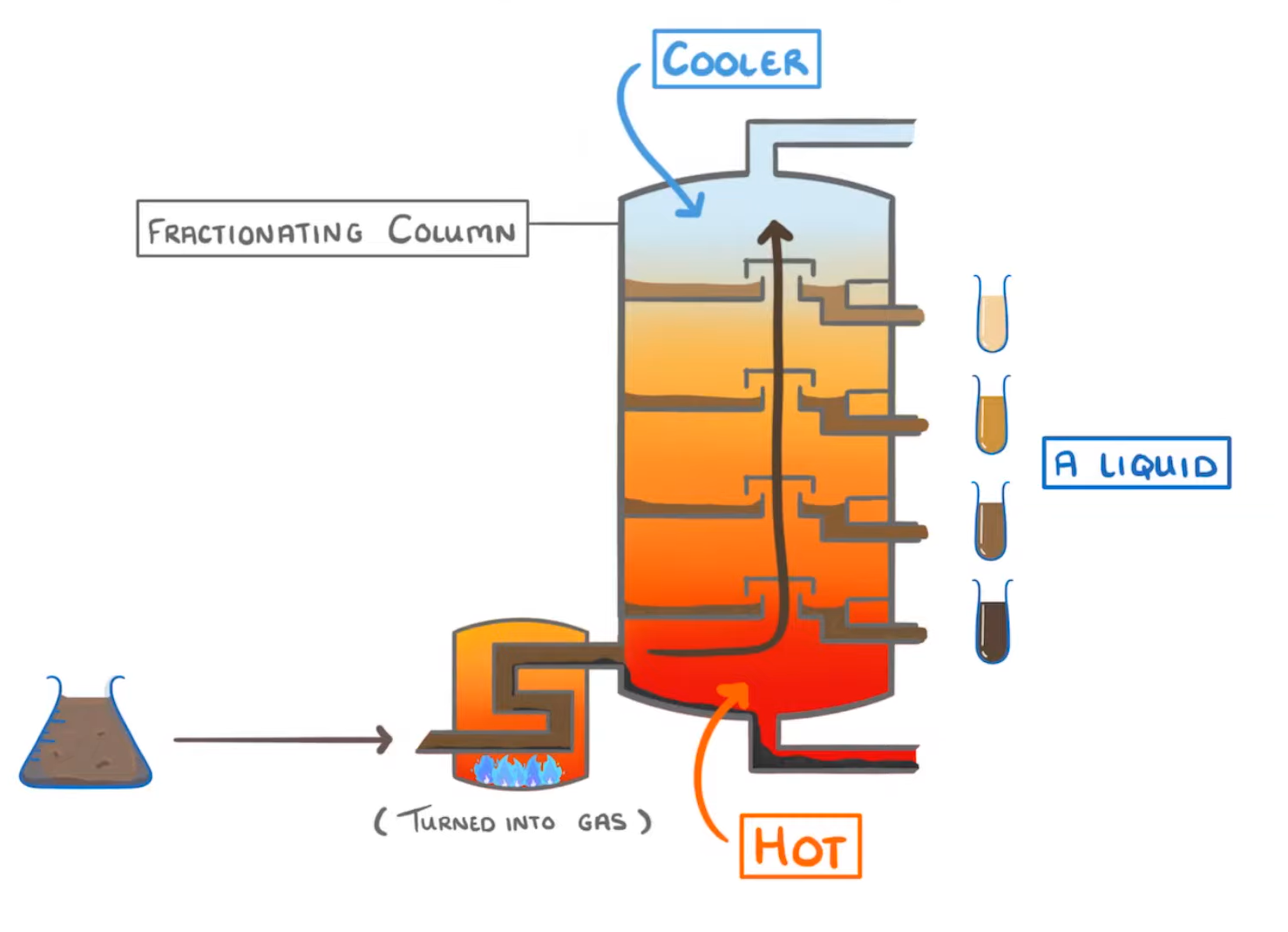
How fractional distillation works:
condense / boiling / cool / warm / longer / shorter / gas / liquid / high / low
- Crude oil is a mixture of hydrocarbons with different points.
- The first step is to heat the crude oil to a very temperature so that all of the compounds are evaporated from liquid to .
- The hot gaseous hydrocarbons then rise up the fractionating column (because hot gas rises).
- As they rise, they down, because the top of the column is cooler than the bottom.
- The hydrocarbons will when they become cooler than their boiling point, and the liquid hydrocarbons then collect in trays and drain out.
- The chain hydrocarbons condense at the bottom of the fractionating column because they have high boiling points.
- Meanwhile the chain hydrocarbons condense at the top of the column because they have much lower boiling points.
|
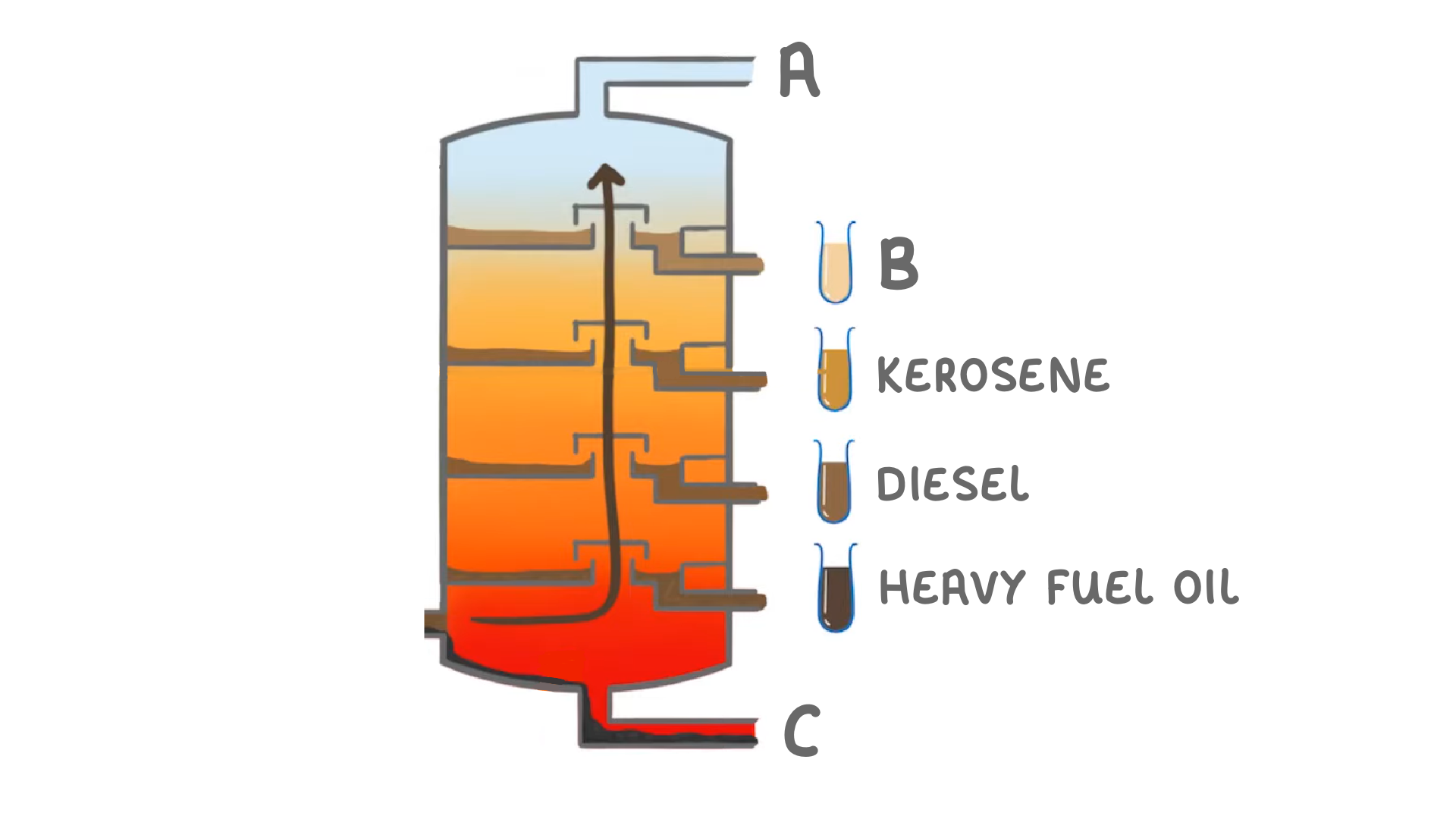
Match the hydrocarbons A to C on the diagram above with the following products:
Petrol:
Liquified Petroleum Gas:
Bitumen:
|
Which of the following hydrocarbons are used as a fuel?
(Select all that apply)
Petrol
Bitumen
Diesel
Kerosene
|
When separating crude oil we use a fractionating column.
Is the top of the column hotter or cooler than the bottom?
Hotter
Cooler
|
Feedstocks and petrochemicals A lot of people get these terms confused, but it's important to know what they mean. |
A feedstock is a raw material used to provide reactants for an industrial reaction. |
A petrochemical is a substance made from crude oil, via chemical reactions. |
So basically, the different hydrocarbons in crude oil are all feedstocks, but the useful things we then make from those hydrocarbons (polymers, solvents, lubricants, detergents etc.), are all petrochemicals. |
You should also know that the collection of industries and companies that are involved in making petrochemicals are known as the 'petrochemical industry'. |
A __________ is a raw material used to provide reactants for an industrial reaction.
petrochemical
hydrocarbon
feedstock
|
A __________ is a substance made from crude oil via chemical reactions.
petrochemical
hydrocarbon
feedstock
|