Alkanes - Properties & Combustion
This lesson covers:
- The properties and trends of alkanes
- The chemical equation for the combustion of alkanes
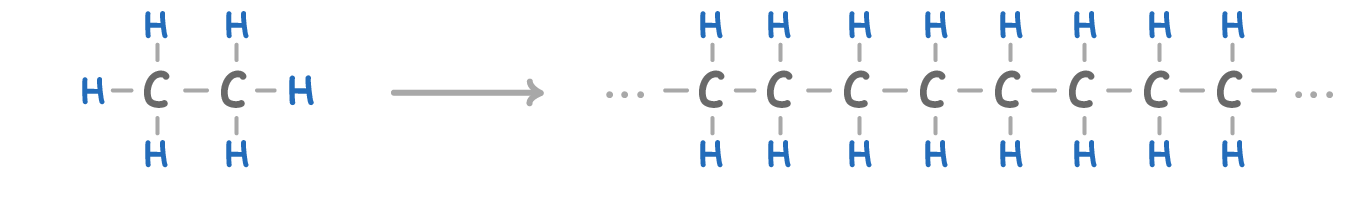 |
As the chain length (number of carbon atoms) of alkanes increases, they become: More viscous Less viscous
|
More volatile Less volatile
|
More flammable Less flammable
|
|
Increasing the chain length (number of carbons) of alkanes leads to:
(Select all that apply)
Lower boiling point
Lower melting point
Higher boiling point
Higher melting point
|
ethanol / oxygen / water / nitrogen
What is the word equation for the complete combustion of a hydrocarbon?
hydrocarbon + ➔ carbon dioxide +
|
C7H16 + ?O2 ➔ ?CO2 + H2O The above combustion reaction needs to be balanced. Start by balancing the number of H2O molecules so that the number of hydrogen atoms is equal on both sides of the equation.
|
C7H16 + ?O2 ➔ CO2 + 8H2O Next, balance the number of CO2 molecules by making sure there are an equal number of carbons on both sides of the equation.
|
C7H16 + O2 ➔ 7CO2 + 8H2O Finish by balancing the number of O2 molecules, so that there are an equal number of oxygen atoms on both sides of the equation.
|
|

Is combustion an exothermic or endothermic reaction?
Exothermic
Endothermic
|
Please balance the following combustion equation:
C3H8 + O2 ➔ CO2 + H2O
|
During a combustion reaction, are carbon and hydrogen oxidised or reduced?
Oxidised
Reduced
|
How to balance an odd number of oxygens To recap, balancing the CO2 and H2O is pretty straight forward once you get the hang of it. You just match the number of carbons and hydrogens from the alkane. C4H10 + ? O2 ➔ 4CO2 + 5H2O |
When balancing the oxygens though, it's common to find an odd number of oxygen atoms on the products side. For example, in the equation above there are 13 oxygens on the products side. To make this balance we would need 6.5O2 on the reactants side (because there are 2 oxygen atom in each molecule and 6.5 x 2 = 13). C4H10 + 6.5O2 ➔ 4CO2 + 5H2O |
In exams though, they prefer you to use whole numbers when balancing equations. The way to resolve these .5 O2 situations is to double everything. So the above equation becomes: 2C4H10 + 13O2 ➔ 8CO2 + 10H2O Now everything is balanced with a whole number of molecules! |
Balance the following combustion equation:
C2H6 + O2 ➔ CO2 + H2O
|