Electrolysis 1 - Introduction
This lesson covers:
- How electrolysis can be used to split compounds
- The equipment required for electrolysis
- How electrons are passed around the circuit
Video Error Notice
In the video, we draw the lines of the battery the wrong way around. Make sure you draw your cells with the shorter lines on the same side as the cathode, as in the correctly drawn diagram below.
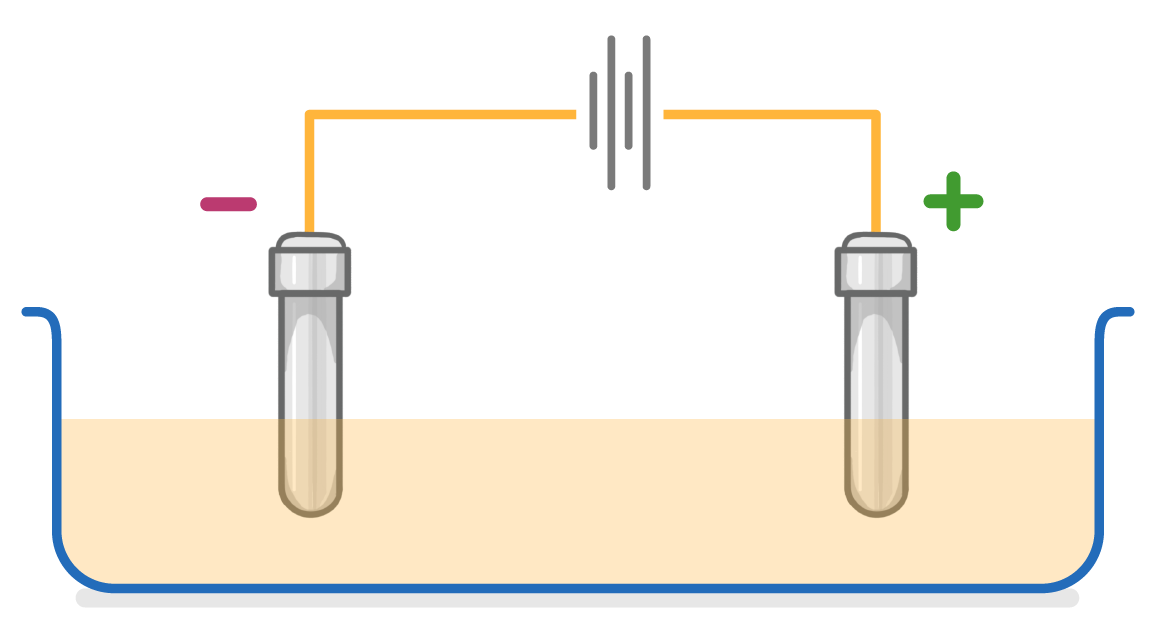
An electrolysis cell has two electrodes. What is the name of the positive electrode?
Cathode
Anode
|
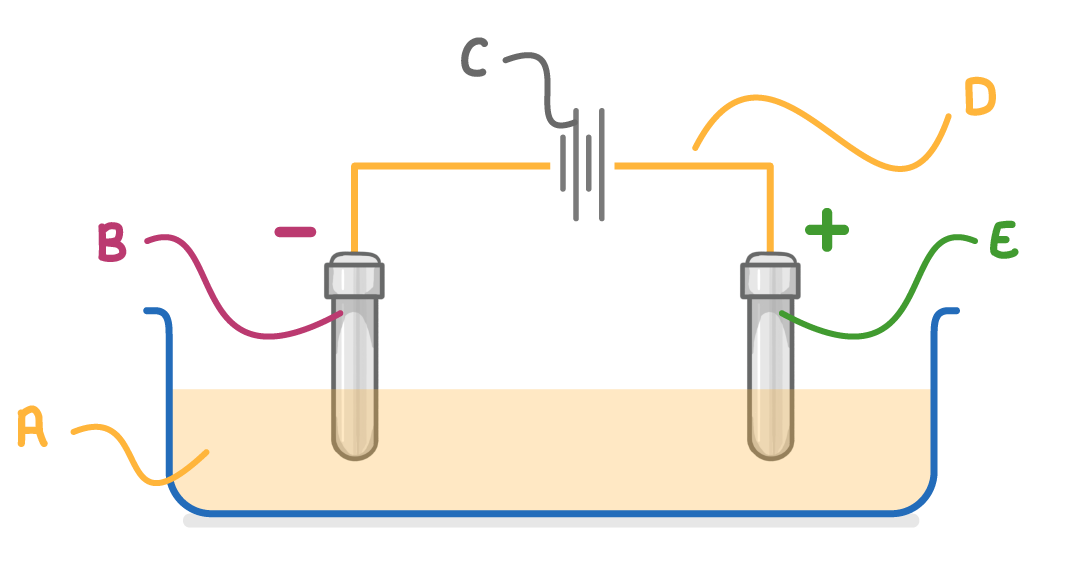
What is the name of the liquid indicated by letter A on the diagram above?
|

In electrolysis, which direction do the electrons travel?
Cathode ➔ Anode
Anode ➔ Cathode
|
Which ions are attracted to the negative electrode (cathode) during electrolysis?
Positive ions
Negative ion
|
The electrodes in an electrolysis cell are normally made of inert carbon. What does the term 'inert' mean?
It is chemically reactive and involved in the reaction
It is unreactive, so will not take place in the reaction.
It cannot conduct electricity
|
In electrolysis, is the ion that gains electrons oxidised or reduced?
Oxidised
Reduced
|
In the electrolysis of molten lead bromide, what is the product at the anode?
Lead
Bromine
Oxygen
|
In terms of electrons, what does oxidation mean?
Gain of electrons
Loss of electrons
|
In electrolysis, why does the compound you're trying to separate need to be molten or dissolved?
|
In electrolysis, which electrode would chloride ions (Cl-) be attracted to?
Cathode
Anode
|
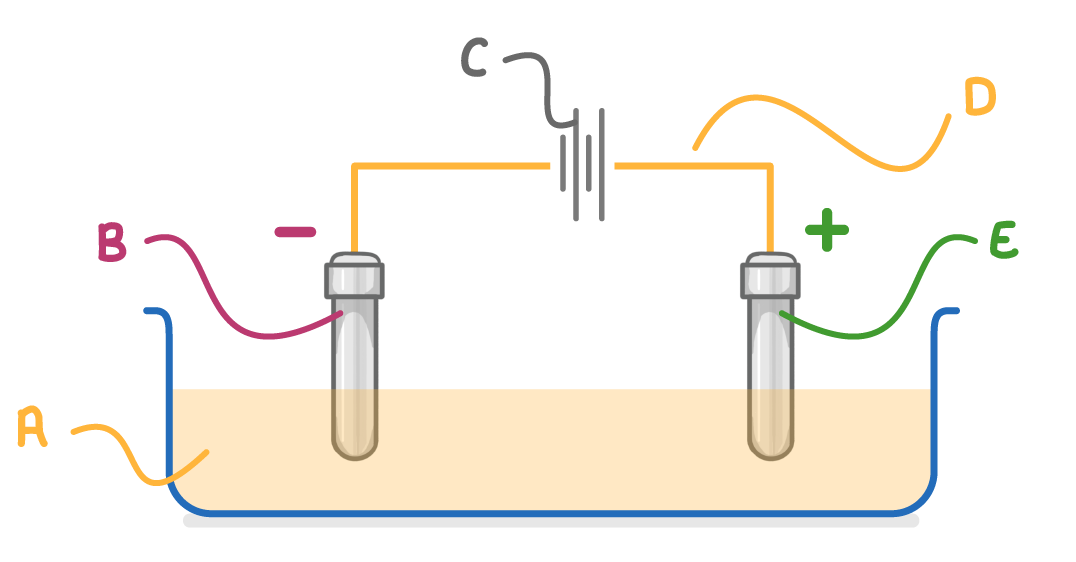
On the diagram above, which letter indicates the anode?
A
B
C
D
E
|