Water of Crystalisation
This lesson covers:
- What 'water of crystallisation' means
- The difference between a 'hydrated' and 'anhydrous' salt
- How to calculate the water of crystallisation of a hydrated salt
What is water of crystallisation?  When salt crystals form they sometimes incorporate water molecules, which we call water of crystallisation. |
 If a crystal does contain water of crystallisation we say that it is hydrated. If it doesn't contain water of crystallisation we say that it is anhydrous. |
The process of adding water of crystallisation is known as 'hydration'. On the other hand, the process of removing water (e.g. by heating) is called 'dehydration'. |
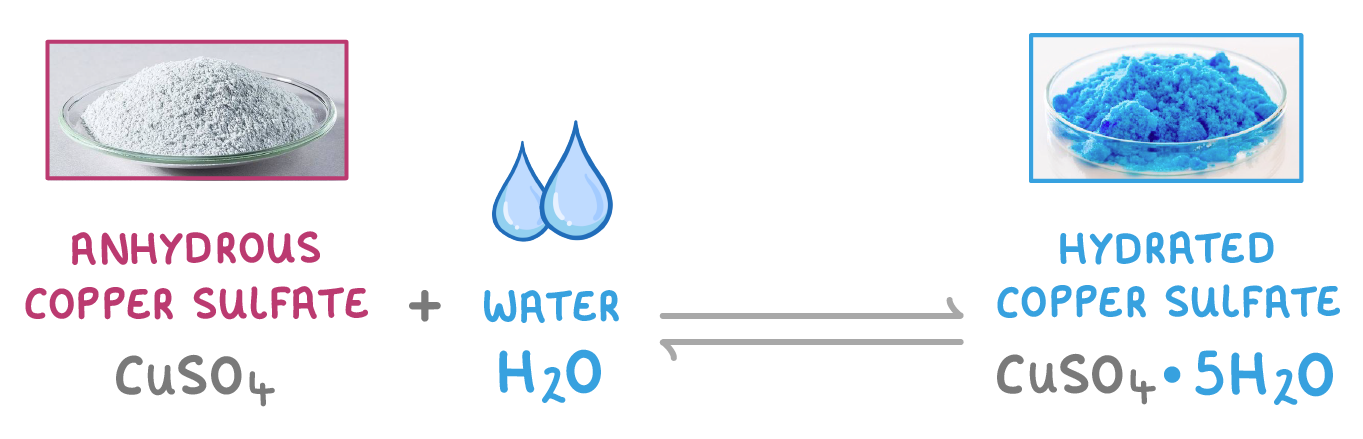 You can see this in copper sulfate. The anhydrous form is white, but if we add some water we can turn it into the hydrated form which is a blue colour. |
We show water of crystallisation by putting a dot followed by the number of water molecules there are. For example, hydrated copper sulfate is CuSO4·5H2O because there are 5 water molecules per CuSO4. |
Recap of key terms:
- A hydrated salt is one in which the solid crystals contain water of crystallisation
- An anhydrous salt is one in which the solid crystals do not contain water of crystallisation
- Hydration is the process of adding water to an anhydrous salt
- Dehydration is the process of removing water from a hydrated salt
- Water of crystallisation refers to the water molecules that are incorporated within a crystal lattice
Worked example: finding the unknown water of crystallisation of a compound
15.67 g of hydrated magnesium carbonate (MgCO3⋅?H2O) was heated to remove the water (H2O). As a result of removing the water, the mass was reduced to 7.58 g. What is the formula of the hydrated compound?
A 4.31 g sample of hydrated Na2CO3 is heated to remove the water (H2O). The mass of the resulting anhydrous compound is 3.22 g. What is the formula of the hydrated compound?
The formula of the hydrated compound is Na2CO3⋅H2O
|
4.92 g of hydrated magnesium sulfate (MgSO4⋅nH2O) was heated to remove the water, producing 2.40 g of anhydrous magnesium sulfate.
Work out the value of n.
The formula of the hydrated compound is MgSO4⋅H2O
|
3.74 g of hydrated copper sulfate is heated to form 2.39 g of anhydrous copper sulfate.
CuSO4⋅nH2O ➔ CuSO4 + nH2O
Work out the value of n.
The formula of the hydrated compound is CuSO4⋅H2O
|
A 1.20 g sample of hydrated tin chloride is heated, causing it to decompose and form 1.01 g of anhydrous tin chloride.
SnCl2⋅nH2O ➔ SnCl2 + nH2O
Calculate n.
The formula of the hydrated compound is SnCl2⋅H2O
|