Diamond & Graphite
This lesson covers:
- The definition of an 'allotrope'
- The structure and properties of diamond and graphite
Two substances made from the same element, that are in the same physical state, but that have different structures, are called .
|
Select two allotropes of solid carbon from this list:
Graphite
Diamond
Silicone dioxide
Carbon dioxide
|
tiny / giant / lattice / cube / carbon / silicon
Diamond and graphite are examples of covalent molecular structures.
Both are made from the element arranged into a large regular repeating .
|
Which of the following statements are true of diamond?
Each carbon atom is bonded to 4 other carbon atoms
Each carbon atom is bonded to 3 other carbon atoms
It conducts electricity
It is made up of silicon and oxygen
|
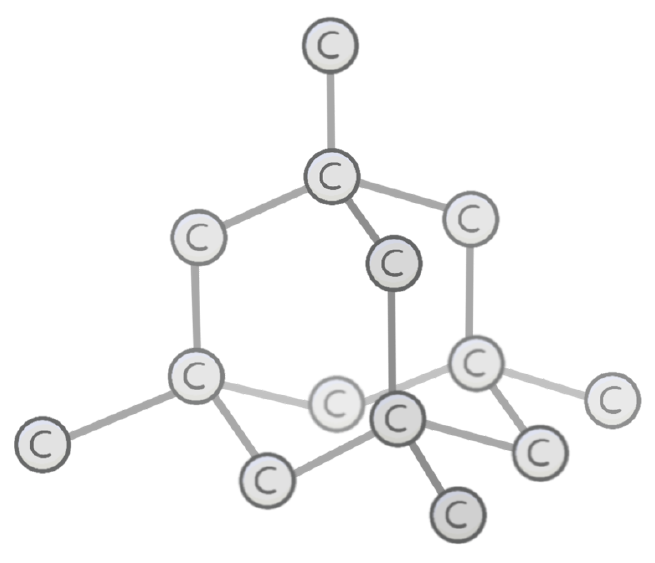
Which structure is shown in the image?
Diamond
Graphite
Carbon
Glass
|
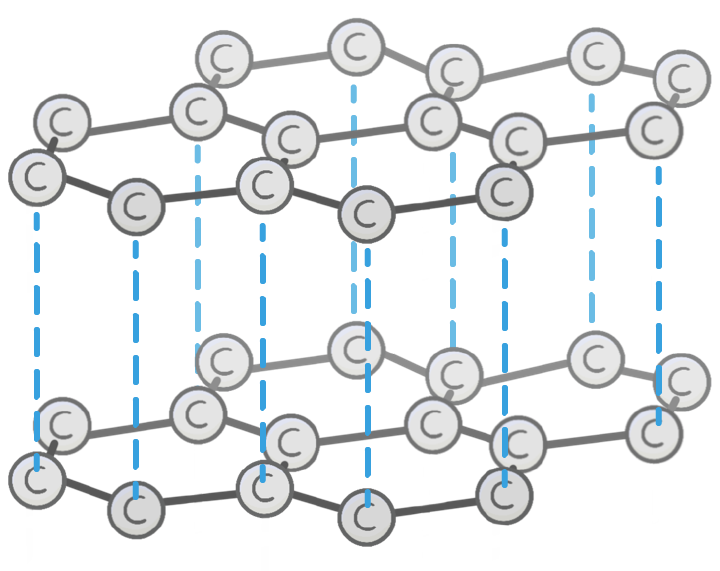
Which structure is shown in the image?
Glass
Carbon
Diamond
Graphite
|
In diamond, each atom is bonded to other atoms via covalent bonds.
This forms a regular 3D lattice which is very strong, and has very melting and boiling points.
|
Can diamond conduct electricity?
Yes
No
|
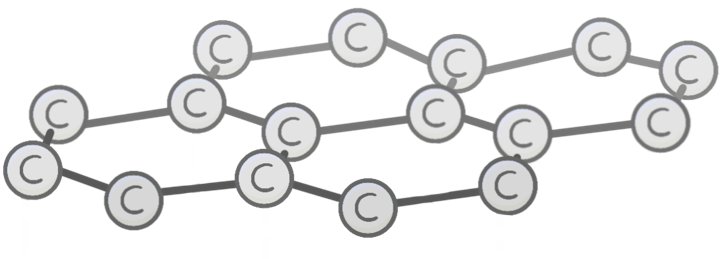
Unlike diamond, the carbon atoms in graphite each bond to other carbon atoms.
This arranges the graphite into 2D layers made up of repeating hexagons.
|
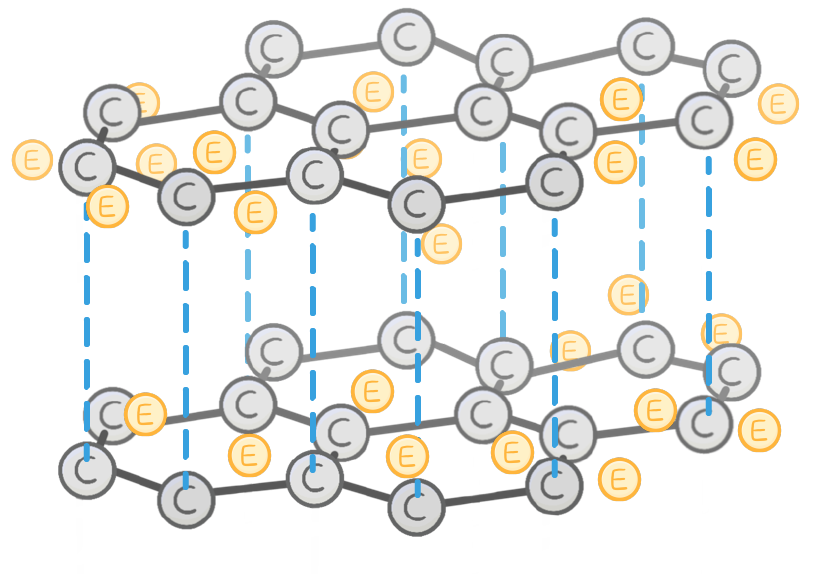
able / unable / localised / delocalised
Graphite is to conduct electricity.
This is because each carbon atom has one electron, which can move freely, and so is able to carry charge.
|
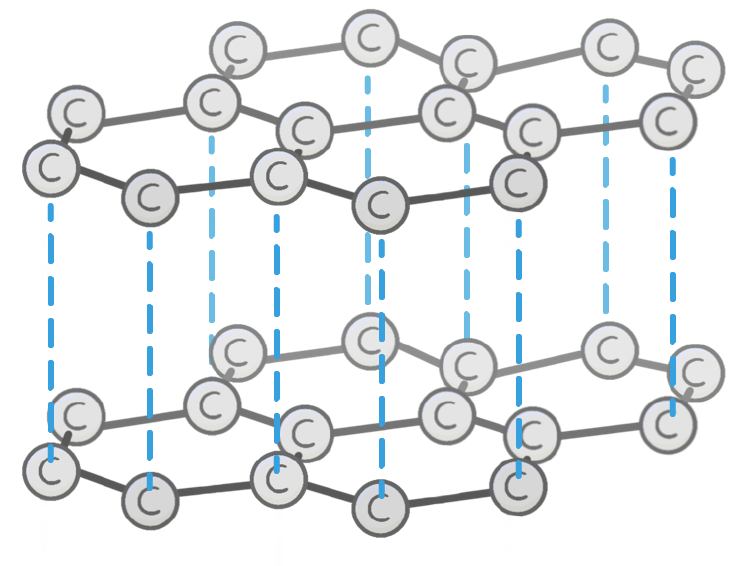
Graphite is a lot softer than diamond.
This is because the 2D layers of graphite stack on top of each other with only weak intermolecular forces holding them together.
This means the layers can slide over each other, making graphite easier to break.