Solutions & Solubility
This lesson covers:
- What the terms solution, solute, solvent and saturated solution mean
- How temperature affects solubility
- Solubility curves
Key terms: |
1Solute - the substance that dissolves in a solvent |
2Solvent - the liquid that the solute dissolves in |
3Solution - the mixture of the dissolved solute and the solvent it is dissolved in |
4Saturated solution - a solution in which the maximum amount of solute has been dissolved, so no more solute will dissolve |
Solubility and temperature |
Solubility is defined as the maximum amount of a substance that will dissolve in a given amount of solvent at a certain temperature. For example, the maximum amount of NaCl that can dissolve in 100g of water (at 25°C) is 35g, therefore the solubility of NaCl is 35g per 100g of water (at 25°C). |
The temperature is important because most substances become more soluble at higher temperatures. We can see this on solubility curves which show how the solubility of a substance changes with temperature. The graph below shows the solubility curve for potassium nitrate (KNO3). |
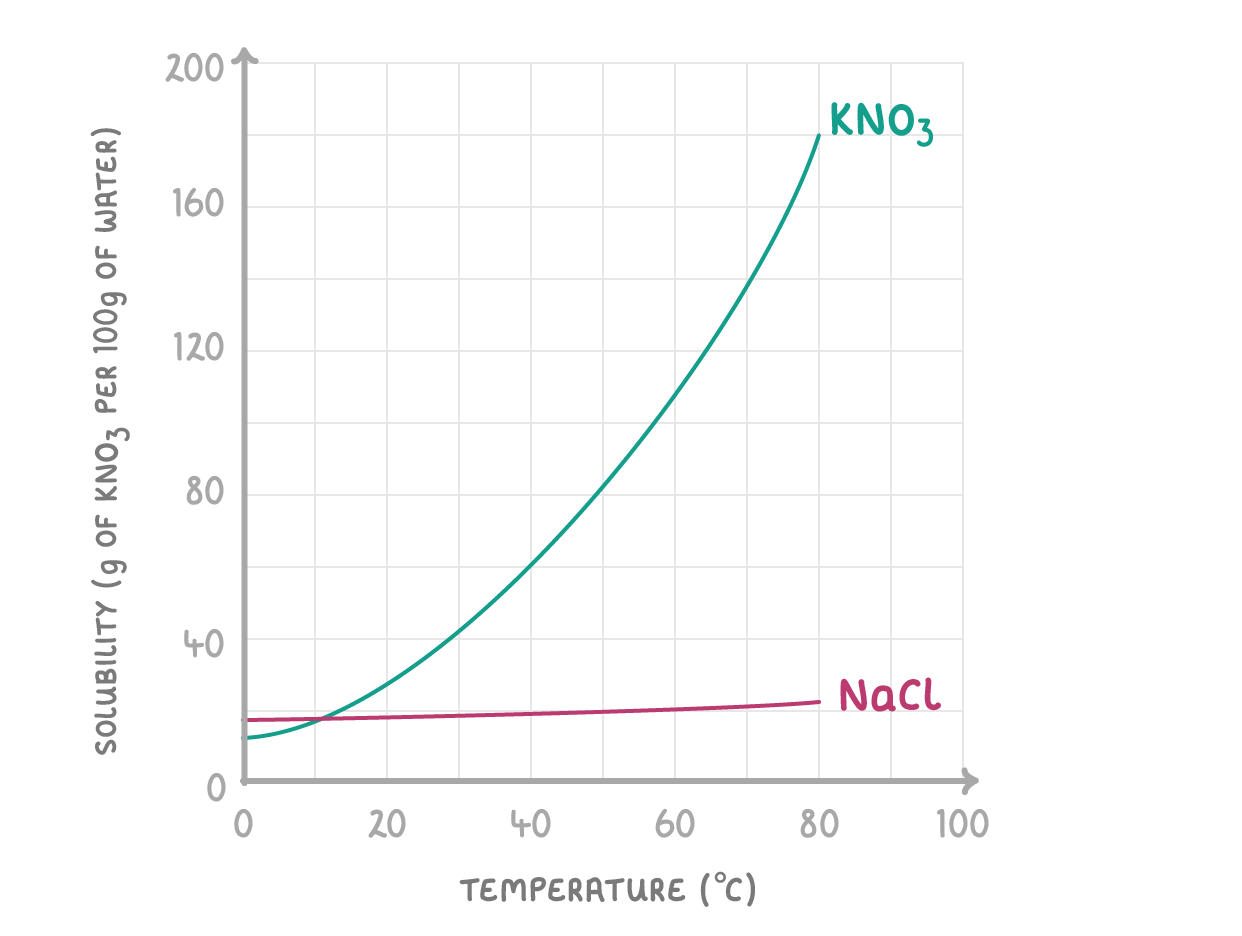 |
Some sodium chloride is dissolved in water.
What do we call the sodium chloride?
Saturated solution
Solution
Solvent
Solute
|
Some potassium nitrate is dissolved in ethanol.
What do we call the ethanol?
Solute
Saturated solution
Solution
Solvent
|
A student keeps adding magnesium chloride to a beaker of water until no more dissolves. At this point we can say that they have a solution.
|
Which units do we usually use to measure solubility?
g/dm3
mol/dm3
g/100g
mol/100g
|
increases / decreases
As the temperature increases, the solubility of most solutes .
|