Gases in the Atmosphere
This lesson covers:
- The composition of gases in the atmosphere
- How magnesium, hydrogen, and sulfur burn in oxygen
- How thermal decomposition of metal carbonates produces carbon dioxide
Composition of the atmosphere |
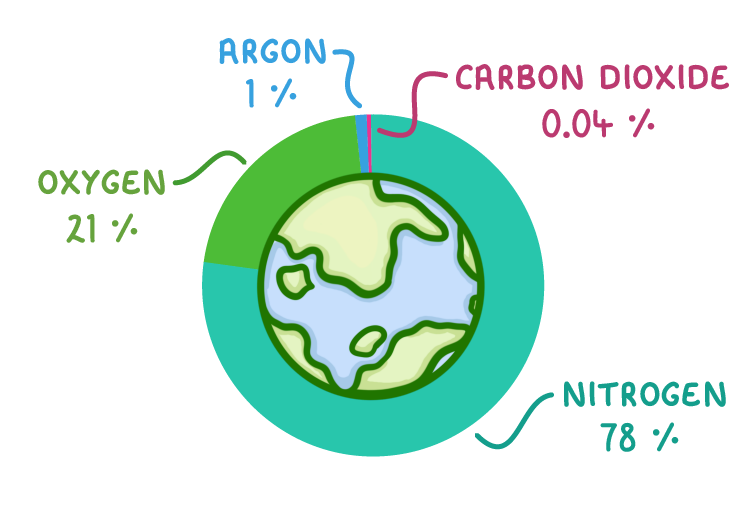 |
The atmosphere is the layer of gases that surrounds the earth. The abundance (amount) of each gas in the atmosphere has changed over time, but at the moment it is almost all nitrogen and oxygen: 78% nitrogen (N2) 21% oxygen (O2) Almost 1% Argon (Ar) 0.04% carbon dioxide (CO2) |
Burning magnesium, hydrogen and sulfur When you burn (combust) something in air, it reacts with the oxygen in the atmosphere to form an oxide. These oxides can be acidic, neutral, or alkaline. |
Magnesium 
|
Hydrogen 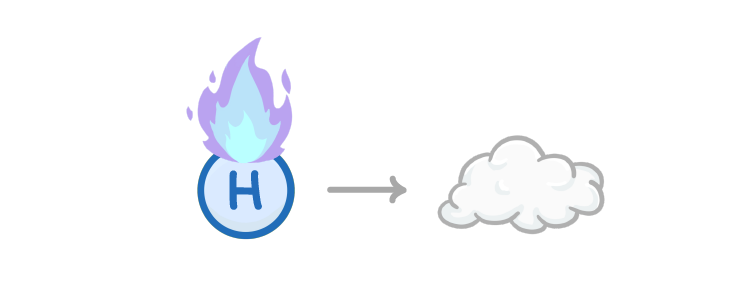
|
Sulfur 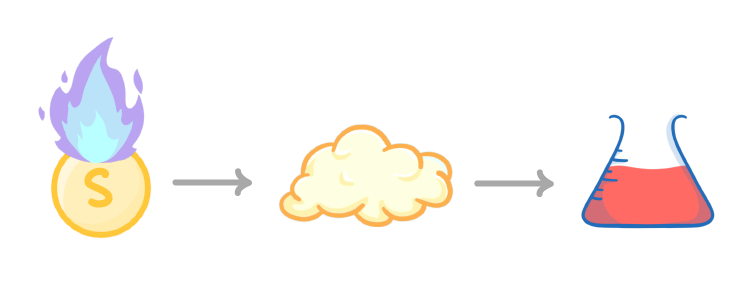
|
Thermal decomposition of metal carbonates produces CO2 |
'Thermal decomposition' means to break something down using heat. This makes sense because thermal refers to heat and decomposition means to decompose or break down. |
When you heat a metal carbonate it will break apart into a metal oxide and carbon dioxide: Metal carbonate ➔ metal oxide + carbon dioxide |
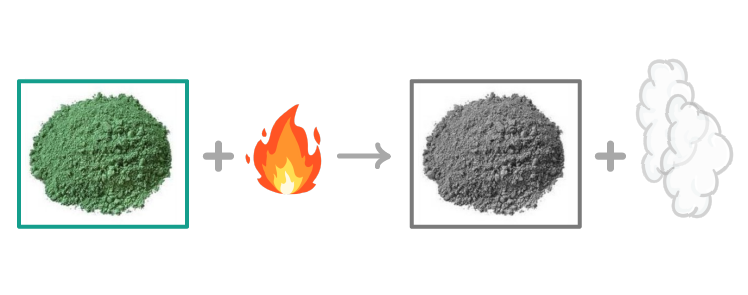 As an example, if you heat copper(II) carbonate (a green powder), it will decompose into copper(II) oxide, which is black, and carbon dioxide, which is a colourless gas: CuCO3(s) ➔ CuO(s) + CO2(g) |
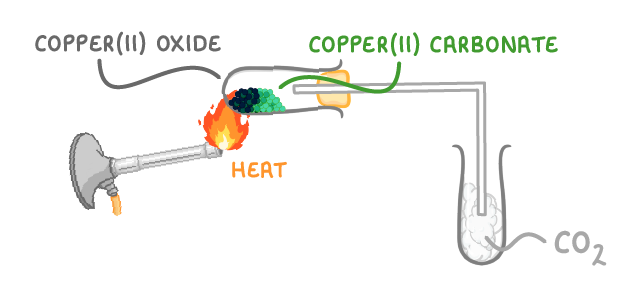 You can see this experimentally by heating copper(II) carbonate and collecting the carbon dioxide that is given off in a test tube. You'll slowly see the powder turn from green to black. |
What percentage of the atmosphere is oxygen?
78 %
1 %
21 %
0.04 %
|
What is the name of the most abundant gas in the atmosphere?
|
What is the percentage abundance of CO2 in the atmosphere?
%
|
What colour flame does magnesium give when burned in oxygen?
Orange/yellow
Pale blue
Purple
White
|

Which substance(s) burn with a pale blue flame?
(Select all that apply)
Magnesium
Sulfur
Hydrogen
|
What does magnesium form when burned in oxygen?
(Give the name in words, not symbols)
|
What does sulfur form when burned in oxygen?
(Give the name in words, not symbols)
|
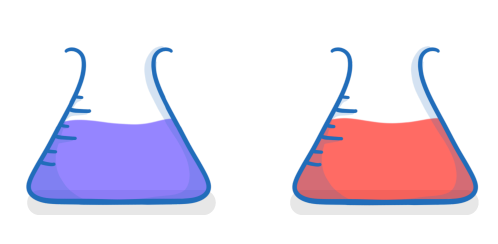
Is magnesium oxide acidic or alkaline when dissolved in water?
Acidic
Alkaline
|
Is sulfur dioxide acidic or alkaline when dissolved in water?
Acidic
Alkaline
|
If you heat a metal carbonate, it will produce a metal and dioxide.
|
What is the colour change when copper(II) carbonate decomposes to copper(II) oxide?
Black ➔ green
Green ➔ black
Brown ➔ green
Green ➔ brown
|