Production of Ethanol
This lesson covers:
- What ethanol is & why it's important
- How ethanol can be produced by fermentation
What is ethanol? |
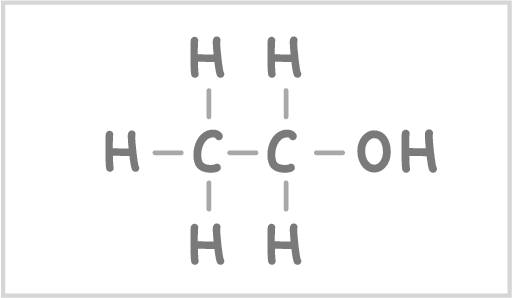 Ethanol is an alcohol with the formula CH3CH2OH |
It has three main uses:
|
Ethanol can be produced from ethene and steam |
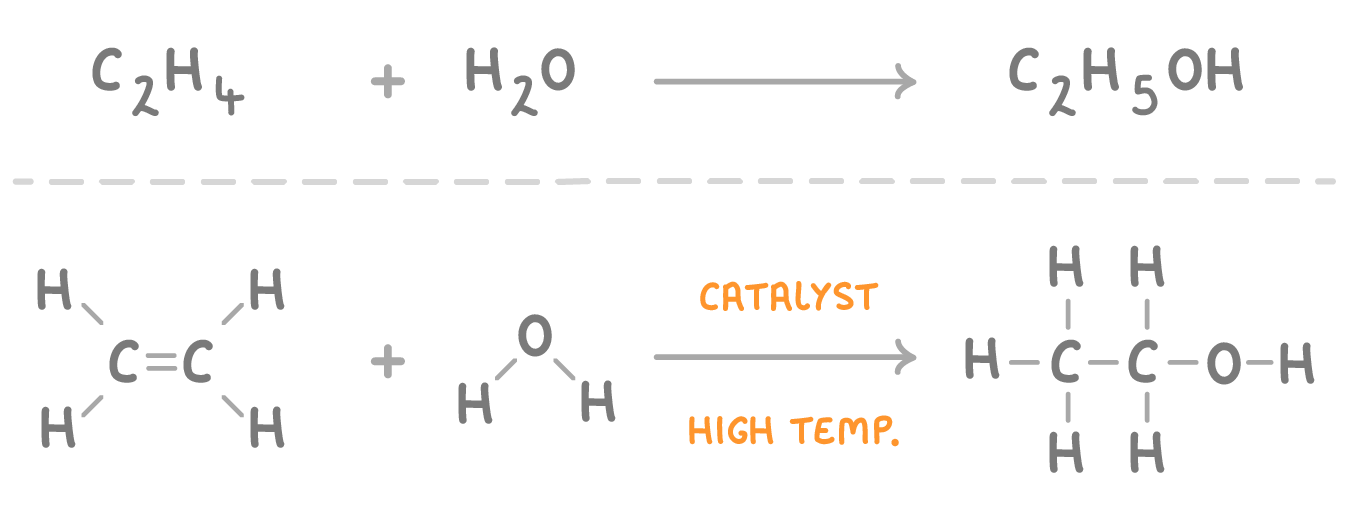 One way that ethanol is produced commercially is to react ethene (C2H4) with steam (gaseous H2O). |
Type of reaction: Addition reaction because the water molecule is being added to the ethene molecule. |
Conditions: High temperature (300 °C), high pressure (60-70 atm), phosphoric acid catalyst. |
Advantages: Ethene is cheap and the reaction itself is cheap and efficient. |
Disadvantages: Ethene is made from crude oil which is a non-renewable resource, so if it starts to run out it will become expensive. |
Ethanol can be produced by fermentation |
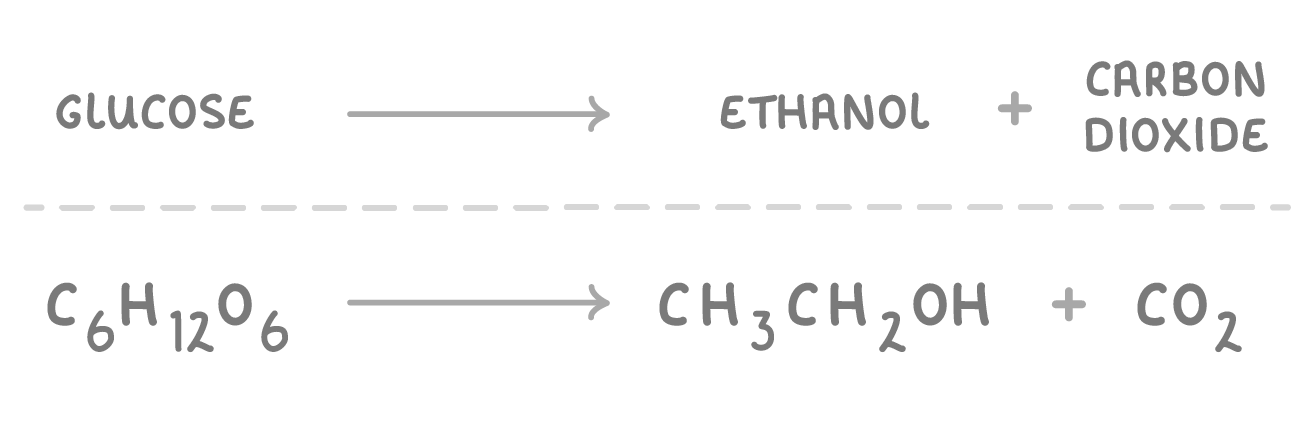 Fermentation is the anaerobic respiration of sugars by yeast cells to produce ethanol and carbon dioxide. |
Type of reaction: Anaerobic respiration (respiration without oxygen). |
Conditions: Carried out in fermentation tanks. Requires yeast cells which have naturally occurring enzymes to catalyse the reaction. Temperatures of 30-40 °C (this is optimum temperature for the enzymes). Must be anaerobic conditions (no oxygen), so that the ethanol isn't oxidised to ethanoic acid. |
Advantages: The sugar/glucose used is a renewable resource so can't run out. Yeast are easy to grow. |
Disadvantages: The process can be relatively slow. The ethanol produced isn't pure so must be distilled by fractional distillation. |

Give 3 uses of ethanol.
|
Ethanol can be produced by fermentation.
Why are temperatures of 30-40 °C used?
This is the optimum temperature for the enzymes
Higher temperatures would be too expensive
Higher temperatures would evaporate the ethanol
|
Ethanol can be produced by fermentation.
Write the symbol equation for this reaction.
|