Electroplating
This lesson covers:
- The process of electroplating
- The reasons why some metal objects are electroplated
What is electroplating? Electroplating uses the principles of electrolysis to cover materials with a layer of metal. |
Example 1 - Improving the appearance of metals  Cheaper, less visually appealing, metals can be covered with more desirable materials such as silver or gold. This process makes jewellery cheaper by not requiring the entire item to be made from precious metal. |
Example 2 - Making metals resistant to corrosion 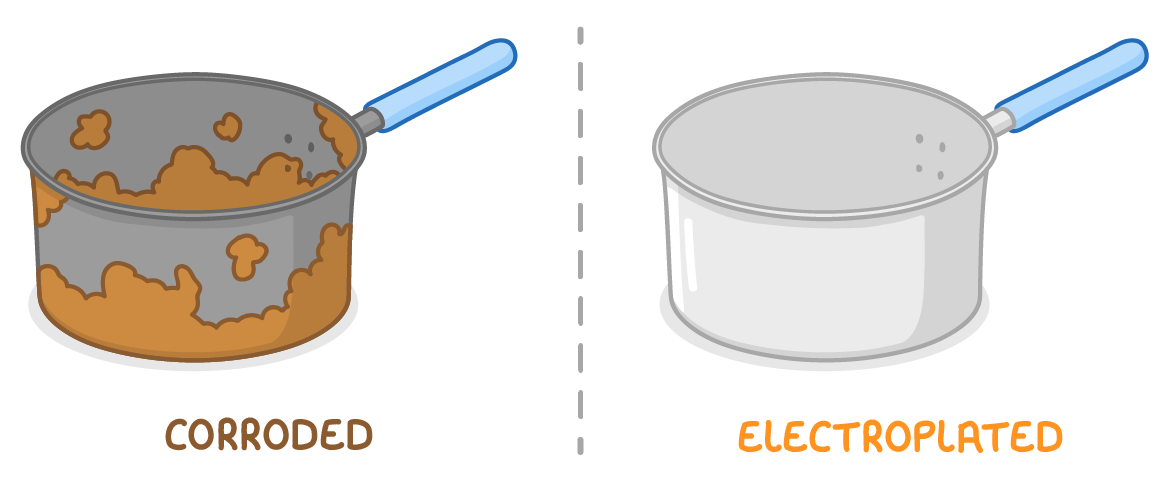 Adding a layer of metal allows the original metal to be covered, reducing exposure to air and water. This adds a layer of sacrificial protection and prevents corrosion. |
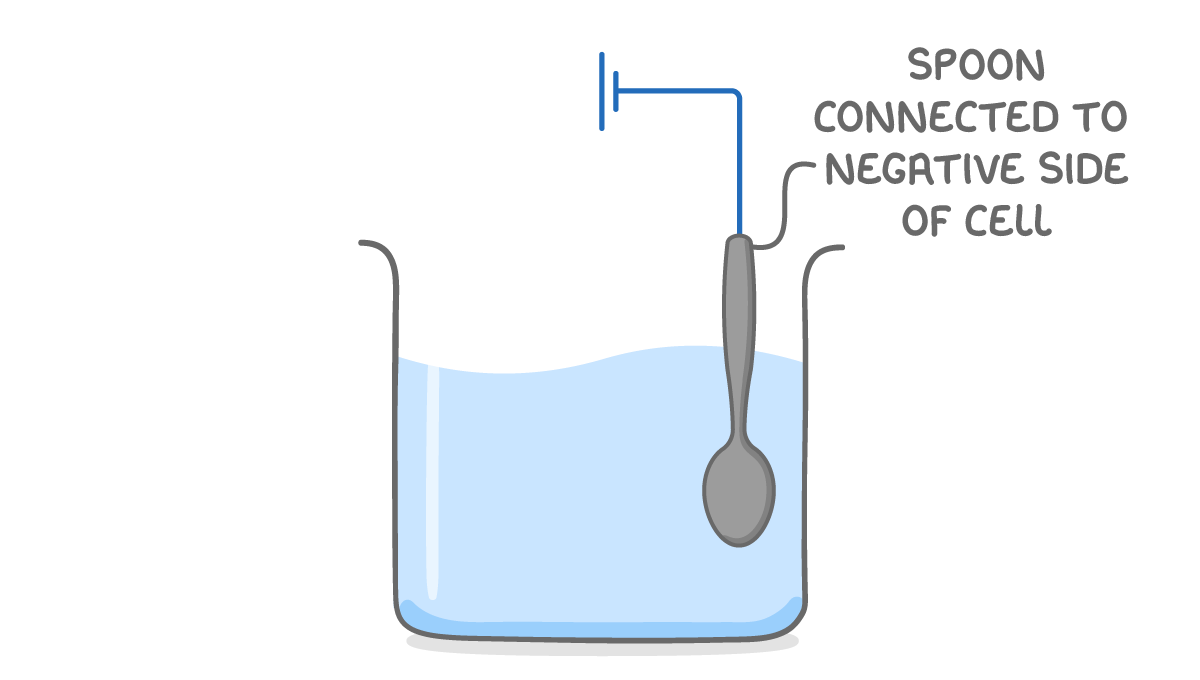
Process of electroplating Cutlery is electroplated to improve its appearance and to prevent corrosion. In this example we will look at electroplating a spoon with silver. |
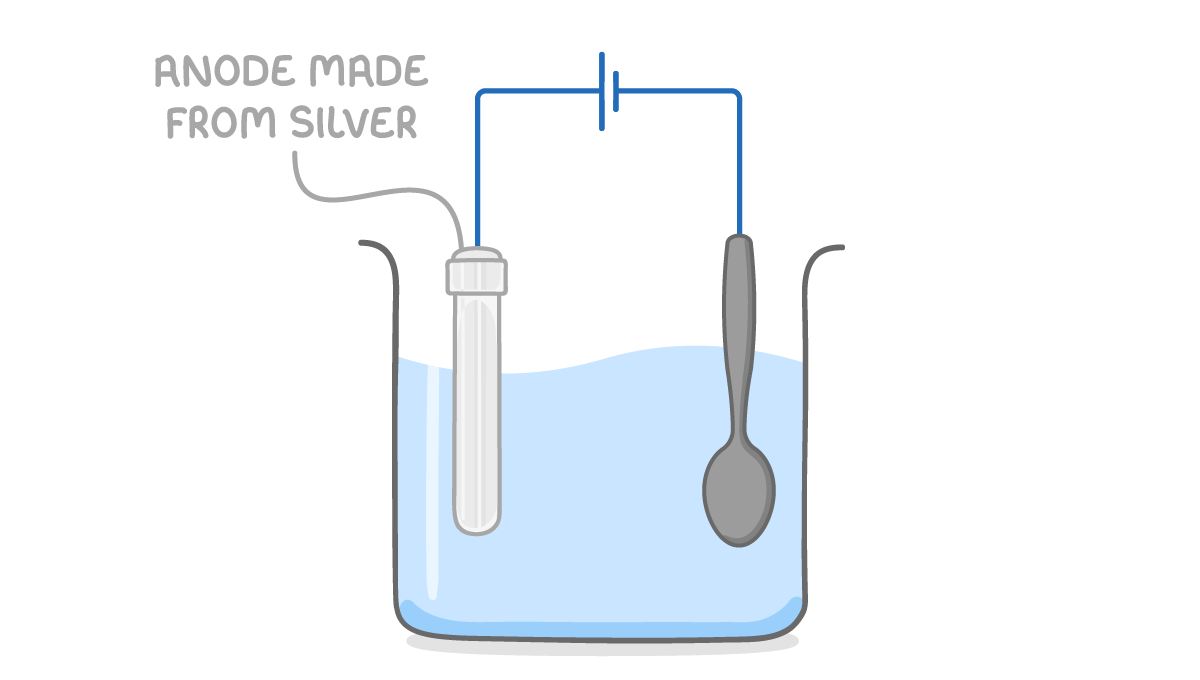
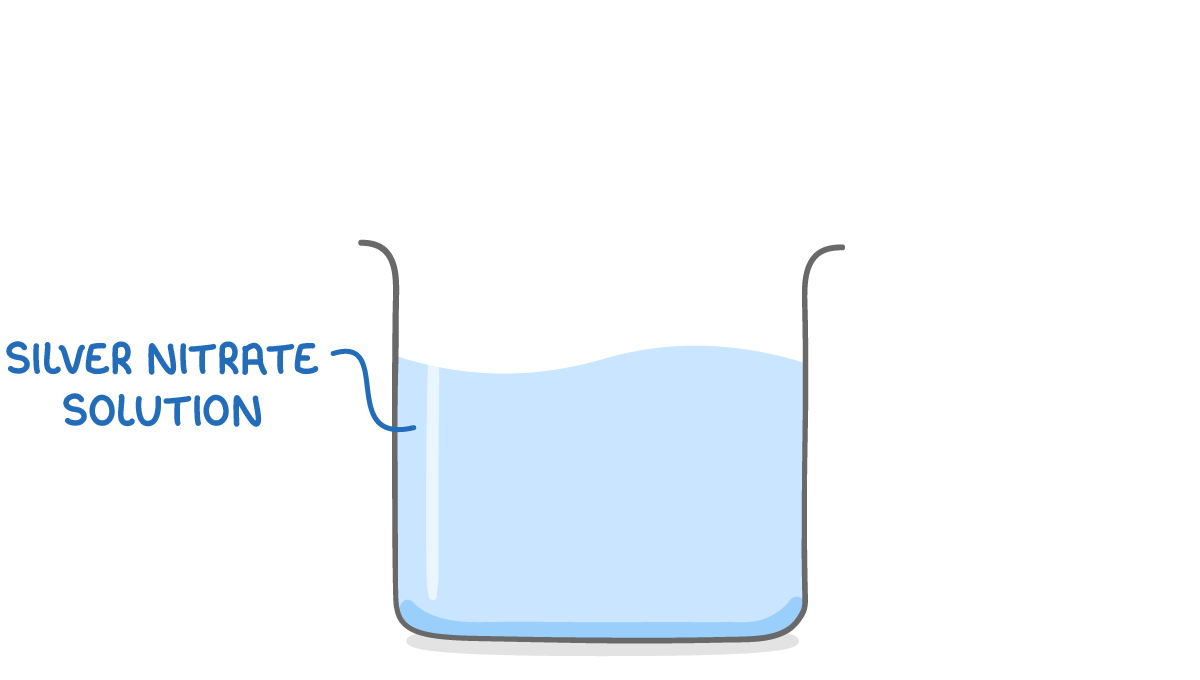 Step 1: Add the electrolyte The electrolyte should be a solution of the plating metal such as silver nitrate solution. |
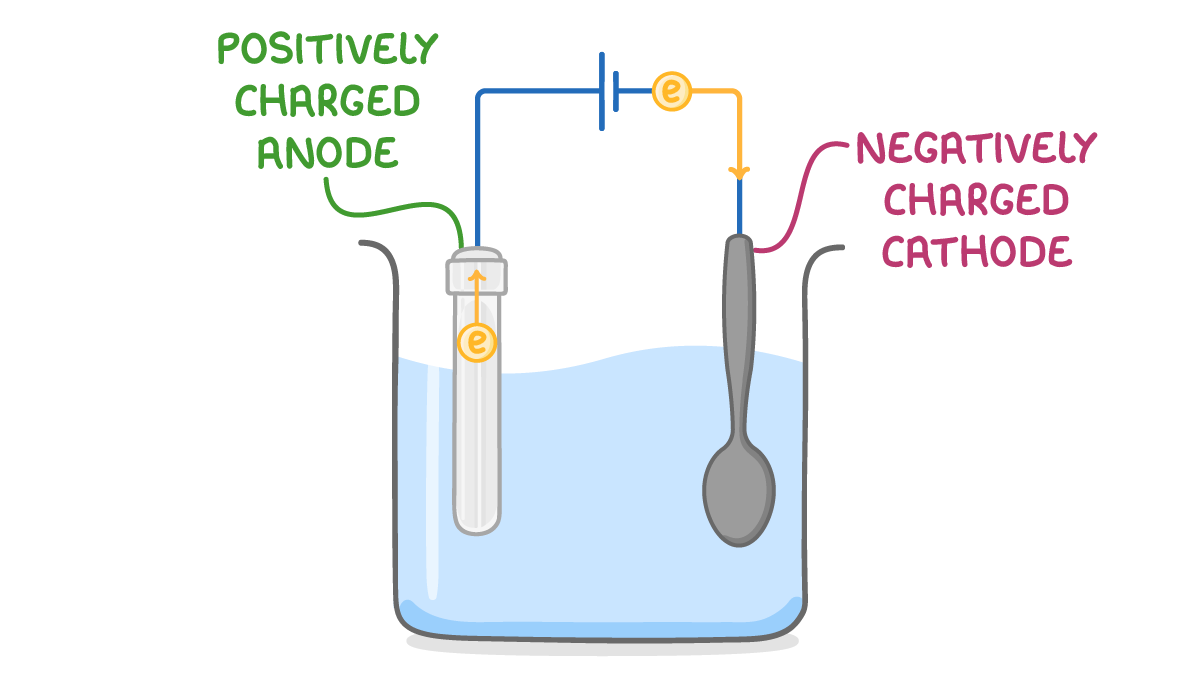
Half equations You need to know the half equations for the reactions at the anode and cathode. |
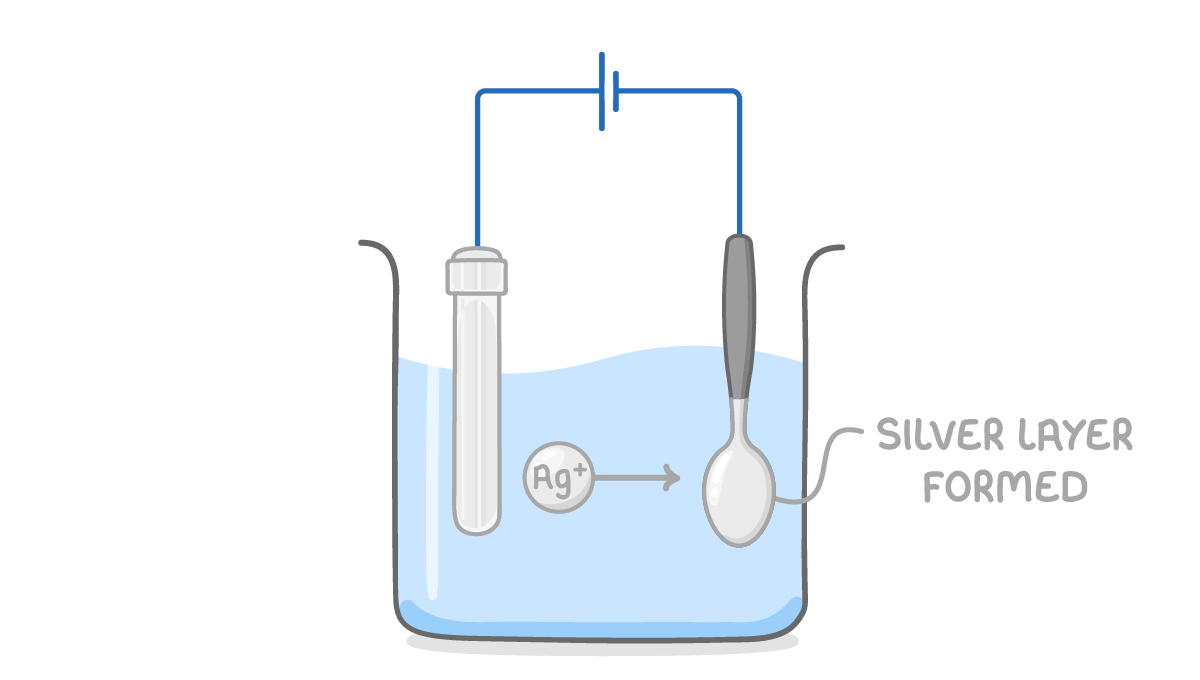
Half equation at cathode: Ag+ + e- ➔ Ag Half equation at anode: Ag ➔ Ag+ + e- |
What happens to the metal at the anode?
it gains electrons to form positive ions
it loses electrons to form negative ions
it gains electrons to form negative ions
it loses electrons to form positive ions
|
What is the purpose of electroplating cutlery?
to improve its appearance
to reduce its weight
to prevent corrosion
to increase its strength
|
Which electrode is the object being electroplated connected to?
negative anode
negative cathode
positive cathode
positive anode
|
What is electroplating?
the process of removing metal from an object using electricity
the process of heating metal to a high temperature to change its properties
the process of shaping metal into different forms using molds
the process of coating an object with a thin layer of metal using electricity
|
Which of the following is NOT an application of electroplating?
removing rust from metal surfaces
decorative coatings on jewellery and silverware
protective coatings on car parts to prevent corrosion
|
What charge do metal ions have?
negative
positive
|