Titrations
This lesson covers:
- What a titration is
- The equipment used in a titration
- The steps in carrying out a titration
- The indicators used in titrations
What is a titration?
A titration is an experimental technique used to find an unknown concentration of an acid or an alkali.
Titration equipment
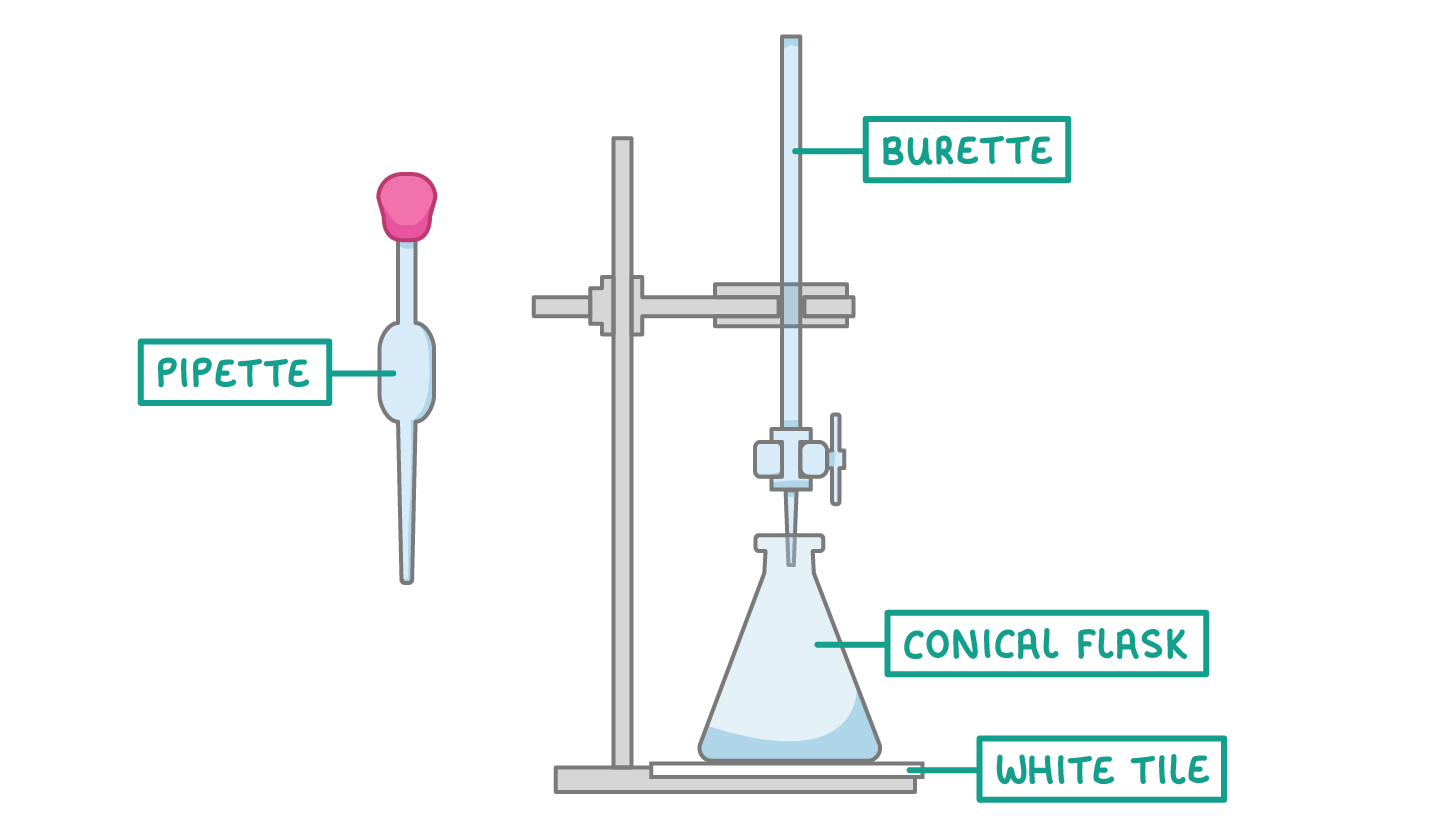
- A pipette to accurately measure a certain volume of acid or alkali (normally 25 cm3)
- A conical flask to contain the liquid from the pipette
- A burette to add alkali or acid to the the conical flask
- A white tile to place the conical flask on
Method The following steps outline how you'd perform a titration in which an acid is added to an alkali: |
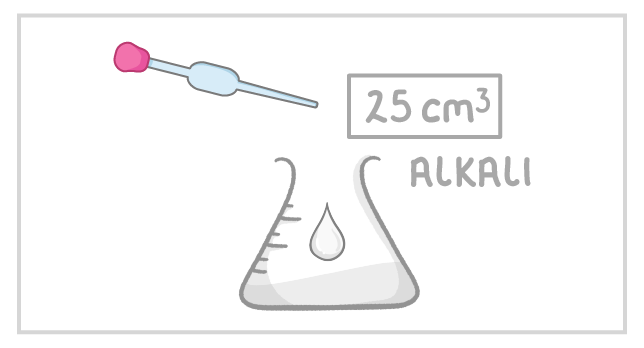 1Use the pipette to add 25 cm3 of alkali to a clean conical flask. |
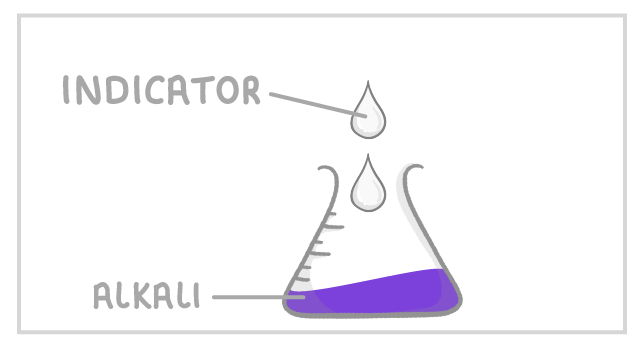 2Add a few drops of indicator and put the conical flask on a white tile. |
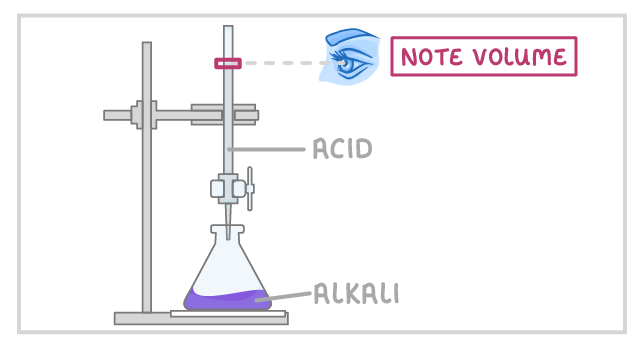 3Fill the burette with acid and note the starting volume. |
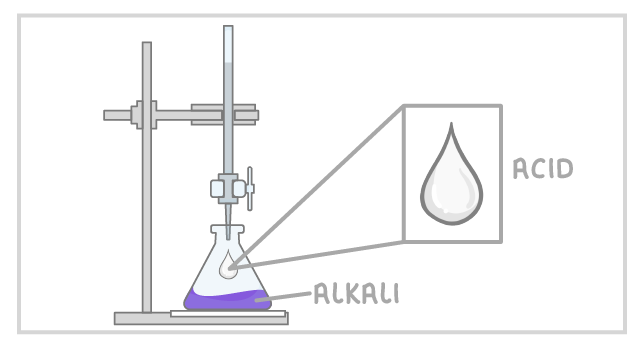 4Slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. |
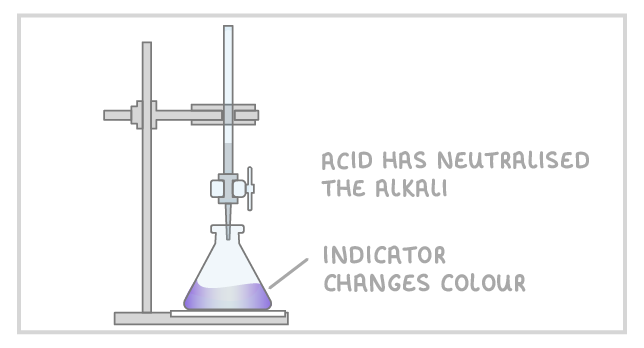 5Stop adding the acid when the end-point is reached (this is when the acid has neutralised the alkali and the indicator changes colour). |
 6Note the final volume reading, and calculate how much acid you added in total. |
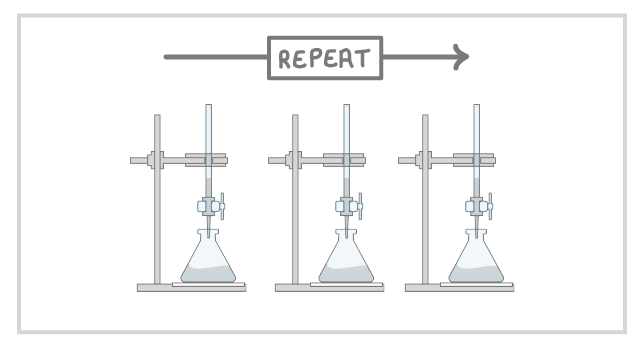 7Repeat the titration until you get 'concordant results', which means volumes of acid that are within 0.10 cm3 of each other. |
8Use the concordant results to calculate the mean volume of acid required to neutralise the alkali. |
Indicators When doing a titration, you must place an indicator in the conical flask so that you can tell when the acid has neutralised the alkali. Indicators show this by changing colour as the pH changes from acidic to alkaline. |
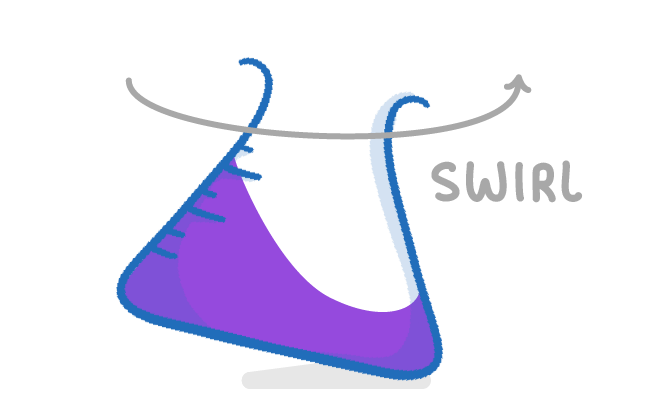 It is important to swirl the conical flask as you add the acid from the burette in order to evenly distribute it, and ensure that the colour change occurs as soon as neutralisation takes place. It's also important that you place the conical flask on a white tile, so you can more easily see when the colour change takes place. |
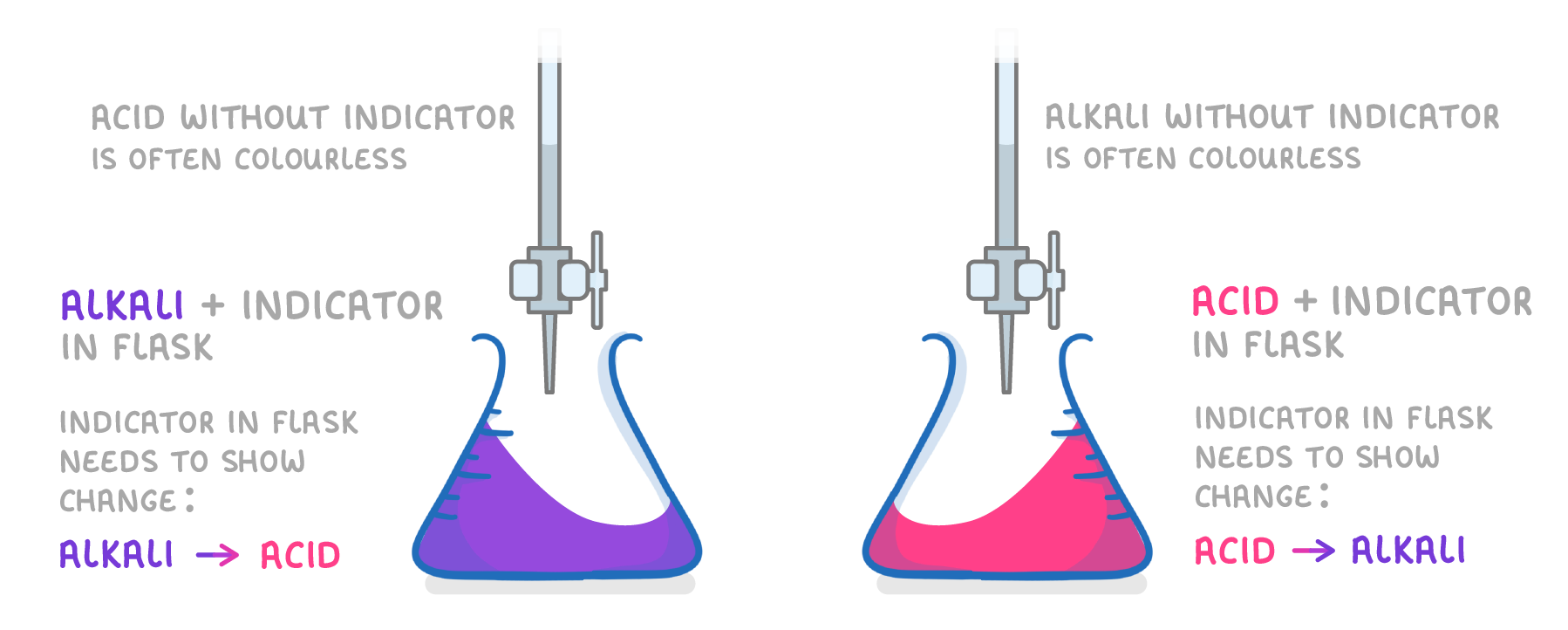 So far in our examples we have talked about adding acid from the burette to an alkali in the flask, but be aware titrations can also be done the other way around, with the acid in the flask, and the alkali in the burette. Because you always add the indicator to the flask (and not the burette), you may need to use a different indicator, depending on whether it's an alkali or acid in the flask. |
There are 3 different type of indicators (and their respective colour changes) you should know: |
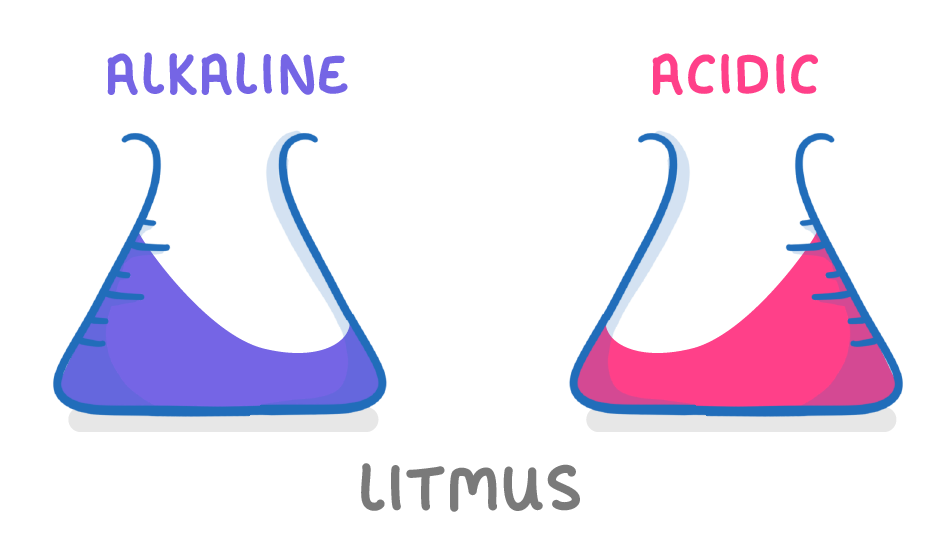 Litmus is red in acidic solutions and blue in alkaline solutions |
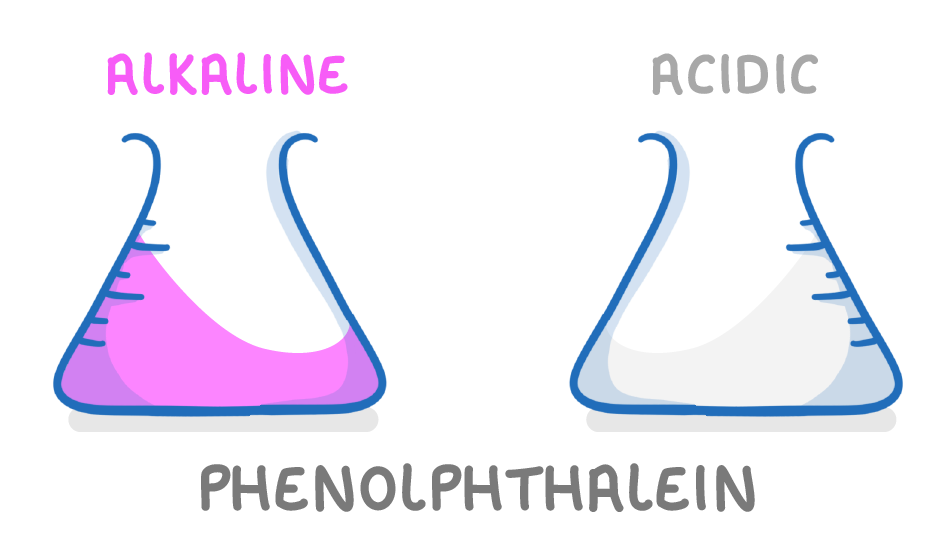 Phenolphthalein is colourless in acidic solutions and pink in alkaline solutions |
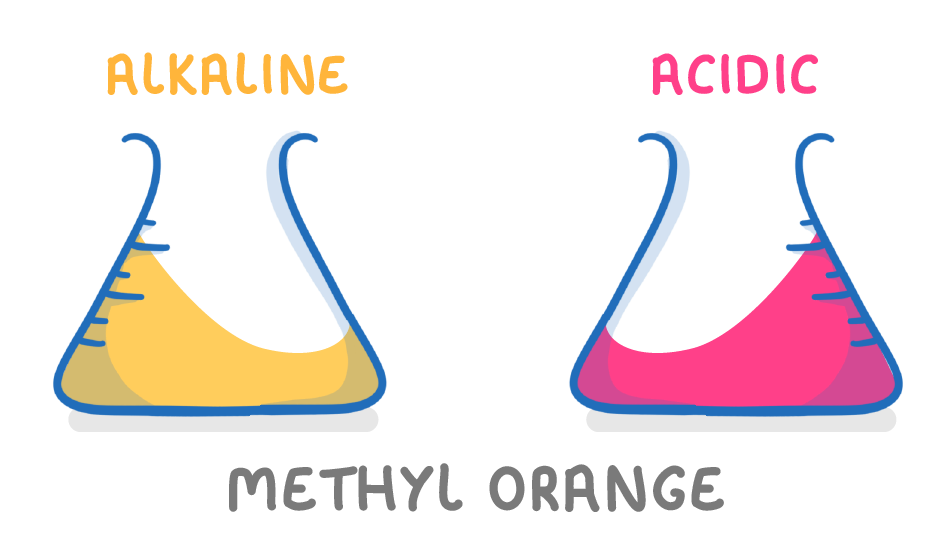 Methyl orange in red in acidic solutions and yellow in alkaline solutions |
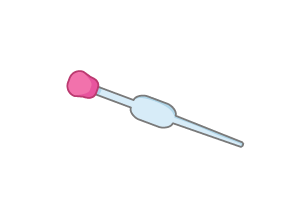
What piece of apparatus is shown in the image above?
|
Which precaution reduces the risk of harm from acid burns?
Tying back long hair
Wearing gloves
Working in a fume cupboard
|
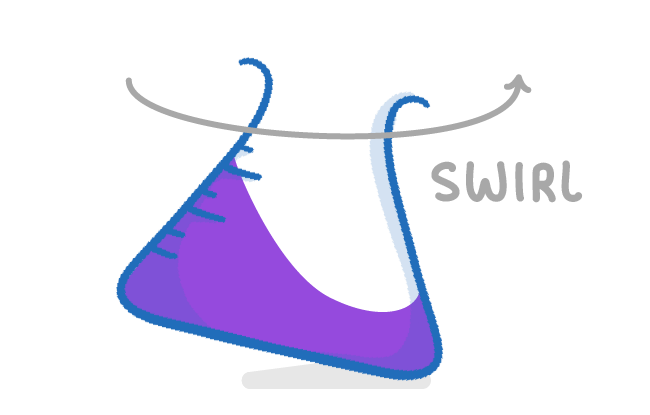
Why should you swirl the conical flask during the titration?
To reduce spilling
To speed up the titration
To evenly distribute the added acid/alkali
|

What piece of apparatus is shown in the image above?
|
The indicator methyl orange is in acidic solutions and in alkaline solutions.
|
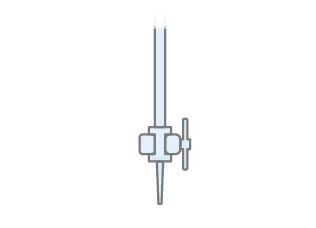
What piece of apparatus is shown in the image above?
|
What colour is phenolphthalein in acidic solutions?
Red
Orange
Pink
Colourless
|
Which piece of apparatus should you use to accurately measure the volume of your acid or alkali before transferring it to a conical flask?
Pipette
Burette
Measuring cylinder
|