Movement of Particles
This lesson covers:
- What diffusion is
- The diffusion of bromine gas in air
- The diffusion of potassium manganate(VII) in water
- The diffusion and reaction of ammonia and hydrogen chloride
Diffusion |
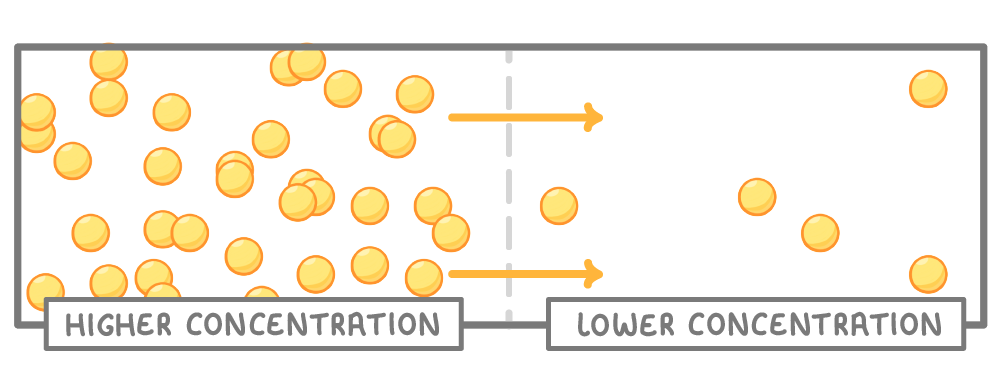 Diffusion is the movement of particles from areas of higher concentration to areas of lower concentration. |
This happens because particles move about randomly, so over time will spread out, moving from where there are lots of them (higher concentration), to where there are fewer of them (lower concentration). |
stronger / weaker / higher / lower / same
Diffusion is the movement of particles from areas of concentration to areas of concentration.
|
Diffusion of bromine gas in air |
 Bromine gas is brown, so we can easily see it spreading out by looking at where the brown colour spreads. |
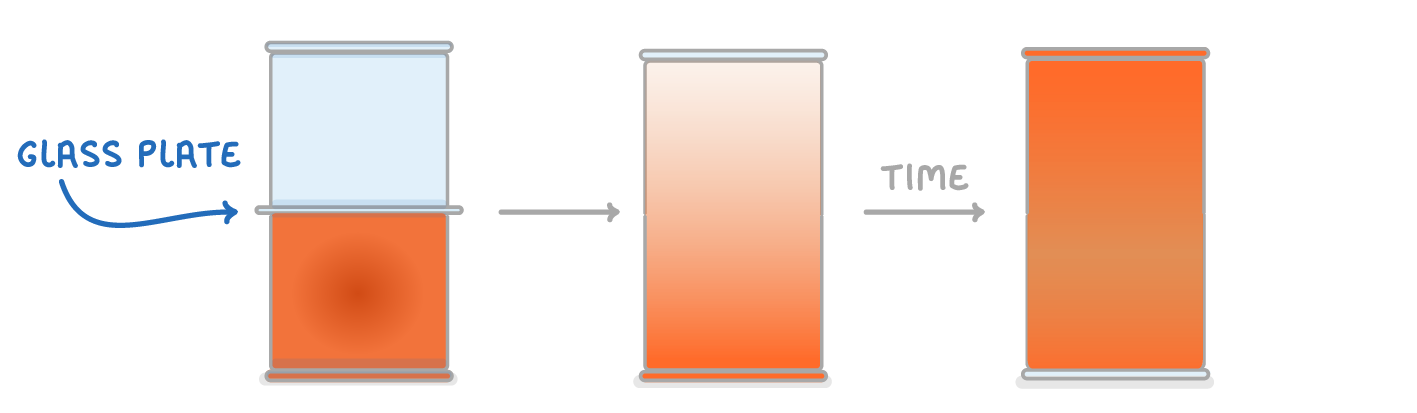 This diagram shows two test tubes that are initially separated by a glass plate. Once the glass plate is removed, the bromine gas diffuses from the bottom tube to the top tube, until in the end it is evenly distributed. |
Diffusion of potassium manganate (VII) in water |
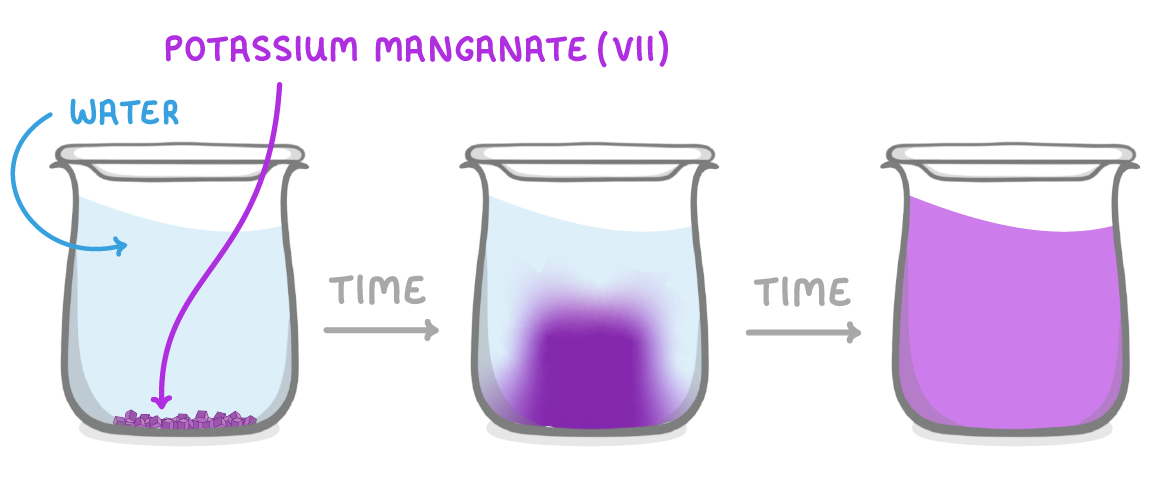 Potassium manganate(VII) is a bright purple colour. If you place a lump of it in water it will initially sink to the bottom. As some of the potassium manganate(VII) particles start to diffuse throughout the beaker of water, the colour will slowly spread out. Eventually, the particles will have diffused evenly throughout the beaker and it will all be the same colour. |
Diffusion and reaction of ammonia and hydrogen chloride: 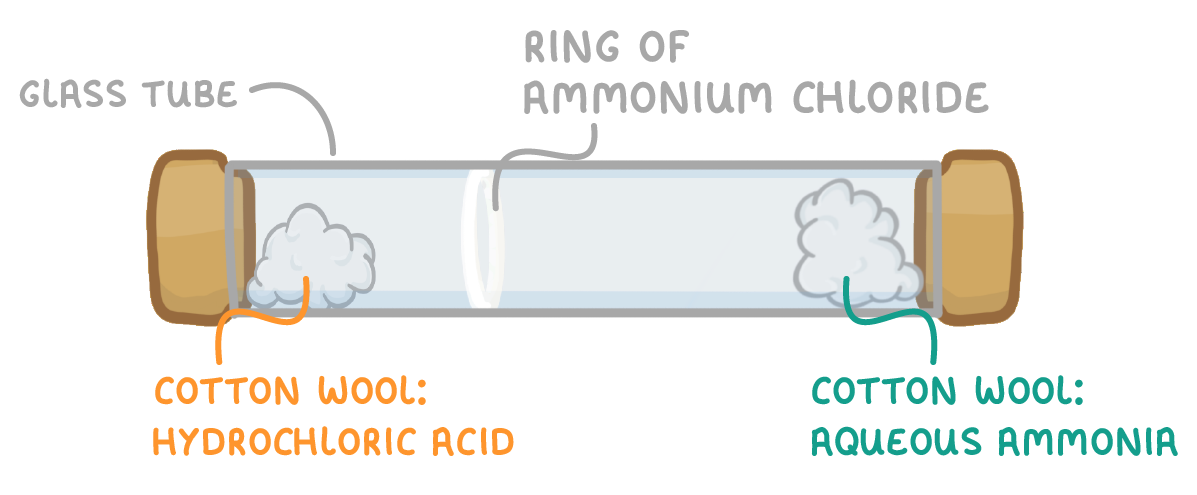
|
What colour is bromine gas?
|
What colour is potassium manganate(VII)?
Brown
Yellow
Purple
Red
|
Are particles of ammonia or particles of hydrogen chloride larger?
Ammonia
Hydrogen chloride
|

Hydrogen chloride gas and ammonia gas react together to form a solid compound.
What is the name of this compound?
|
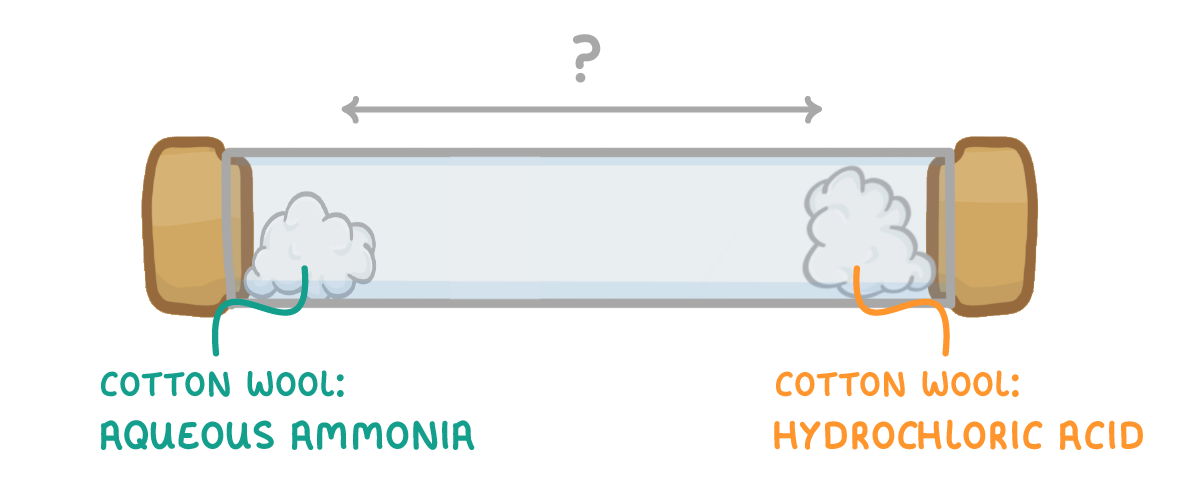
In the diagram, will the ring of ammonium chloride form closer to the cotton wool soaked in ammonia or the cotton wool soaked in hydrochloric acid?
Cotton wool soaked in ammonia
Cotton wool soaked in hydrochloric acid
|