The Haber Process
This lesson covers:
- What 'the Haber process' is & why it's so important
- How the reaction works
- Why the conditions of 450°C and 200 atm are used
Video Error Notice
At 3:05. We say that the hydrogen and nitrogen don't condense because they have 'higher boiling points' than ammonia, but we meant to say that hydrogen and nitrogen don't condense because they have 'lower boiling points' than ammonia. This is why ammonia condenses but hydrogen and nitrogen stay as gases.
Sorry about that, we'll fix it when we update the video.
Which of the following is the reaction of the Haber process?
nitrogen + hydrogen ➔ ammonia
nitrogen dioxide + hydrogen ➔ ammonia + water
nitrogen + oxygen ➔ nitrogen dioxide
|
Balance the the following equation:
N2 + H2 ⇌ NH3
|

Is the Haber process an exothermic or endothermic reaction?
Exothermic
Endothermic
|
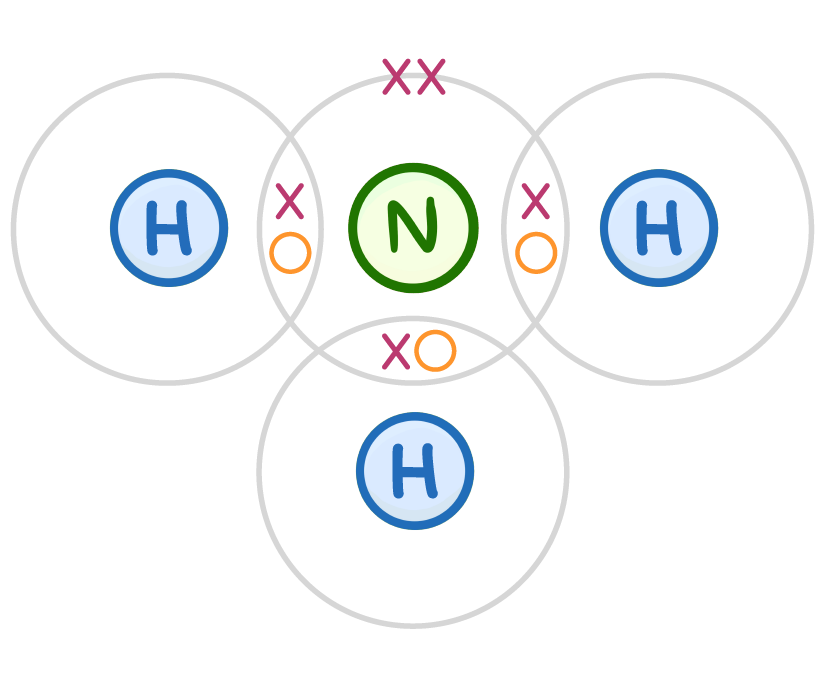
Why is ammonia so important?
It is used in fertilisers
It is used as a fuel
It is used in medicines
|
Which metal acts as a catalyst for the Haber process?
Iron
Aluminium
Nickel
|
Where do we get the nitrogen required for the Haber process from?
Make it from hydrocarbons
Waste product of sewage treatment
Take it from the air
|
Where do we get the hydrogen required for the Haber process from?
Take it from the air
Make it from hydrocarbons
Waste product of sewage treatment
|
What does the '⇌' symbol mean in a chemical equation?
The reaction is very fast
There is not enough energy for the reaction to take place
The reaction is reversible
|
Explain why a temperature of 450°C is used in the Haber process.
|
Explain why a pressure of 200 atmospheres is used in the Haber process.
|