Development of the Periodic Table
This lesson covers:
- The arrangement of the periodic table
- What a 'group' is on the periodic table
- What a 'period' is on the periodic table
The table displays all of the known elements.
A row of elements is called a , while each column is called a .
|
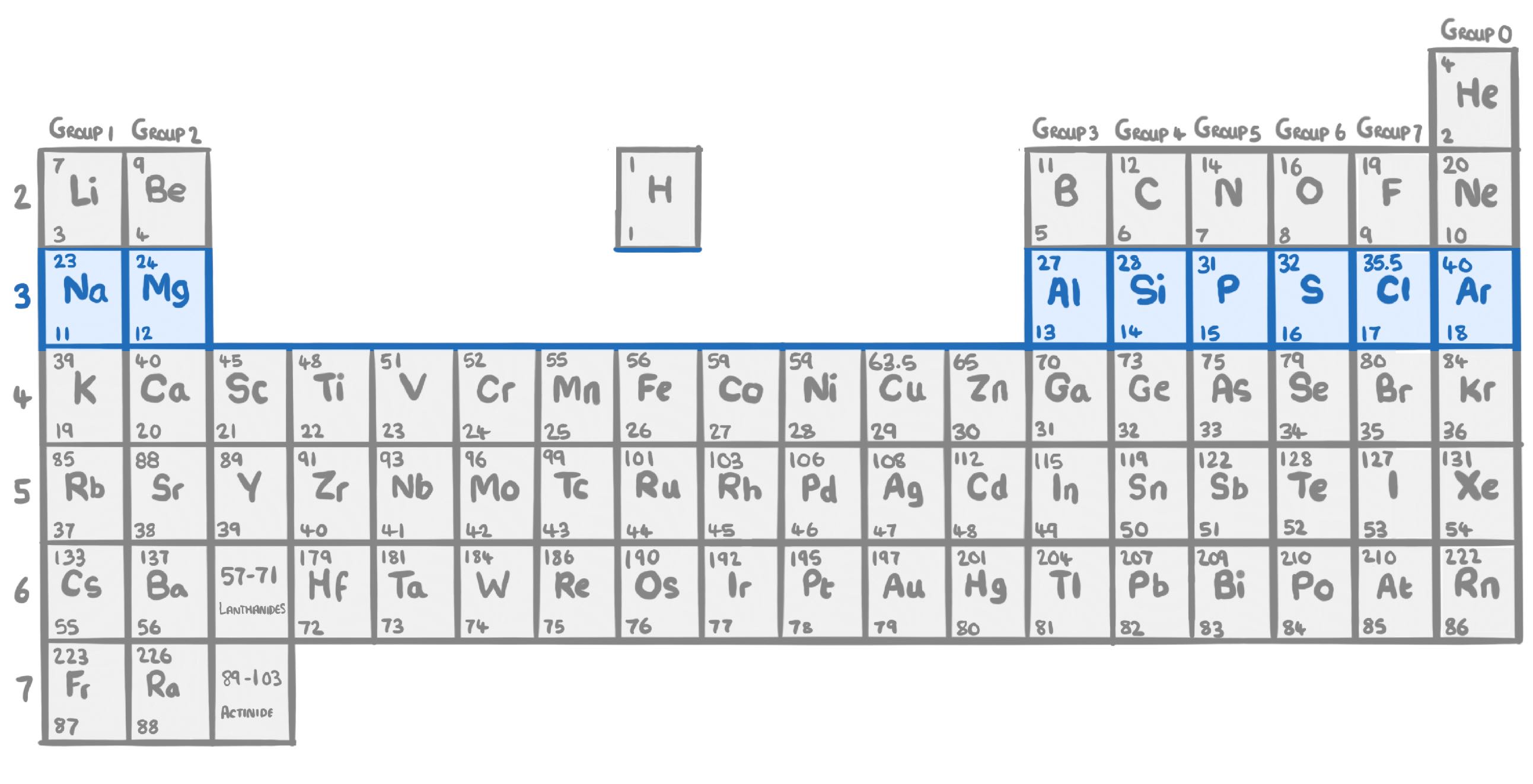
True or false? Elements within a period show similar chemical properties to each other.
True
False
|
The atoms of elements in the same group have the same number of:
Electrons in their outer shell
Electrons
Protons
Shells
|
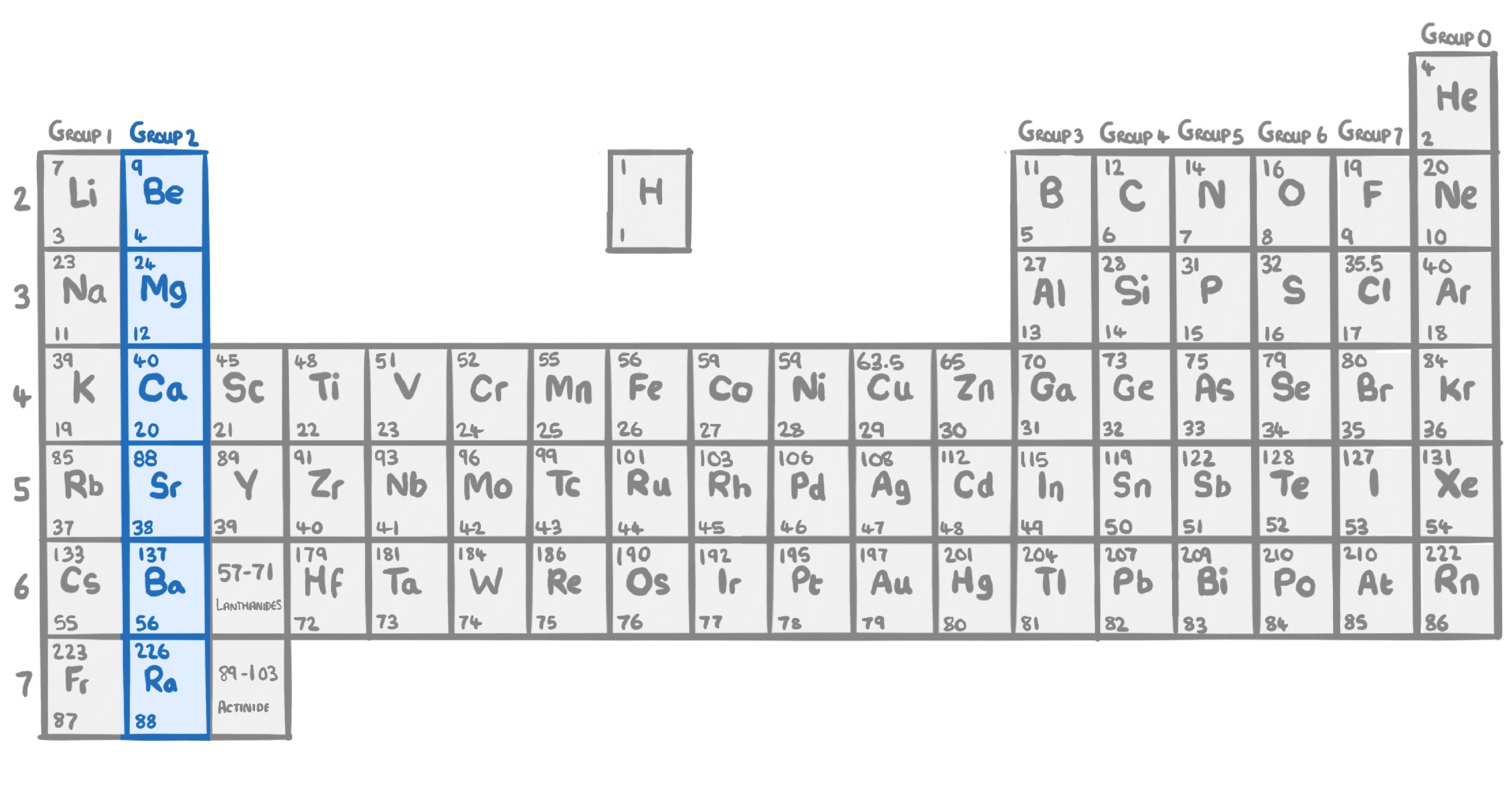
True or false? Elements within a group show similar chemical properties to each other.
True
False
|
The alkali metals in group 1 of the periodic table all have how many electrons in their outer shell?
1
2
3
0
|
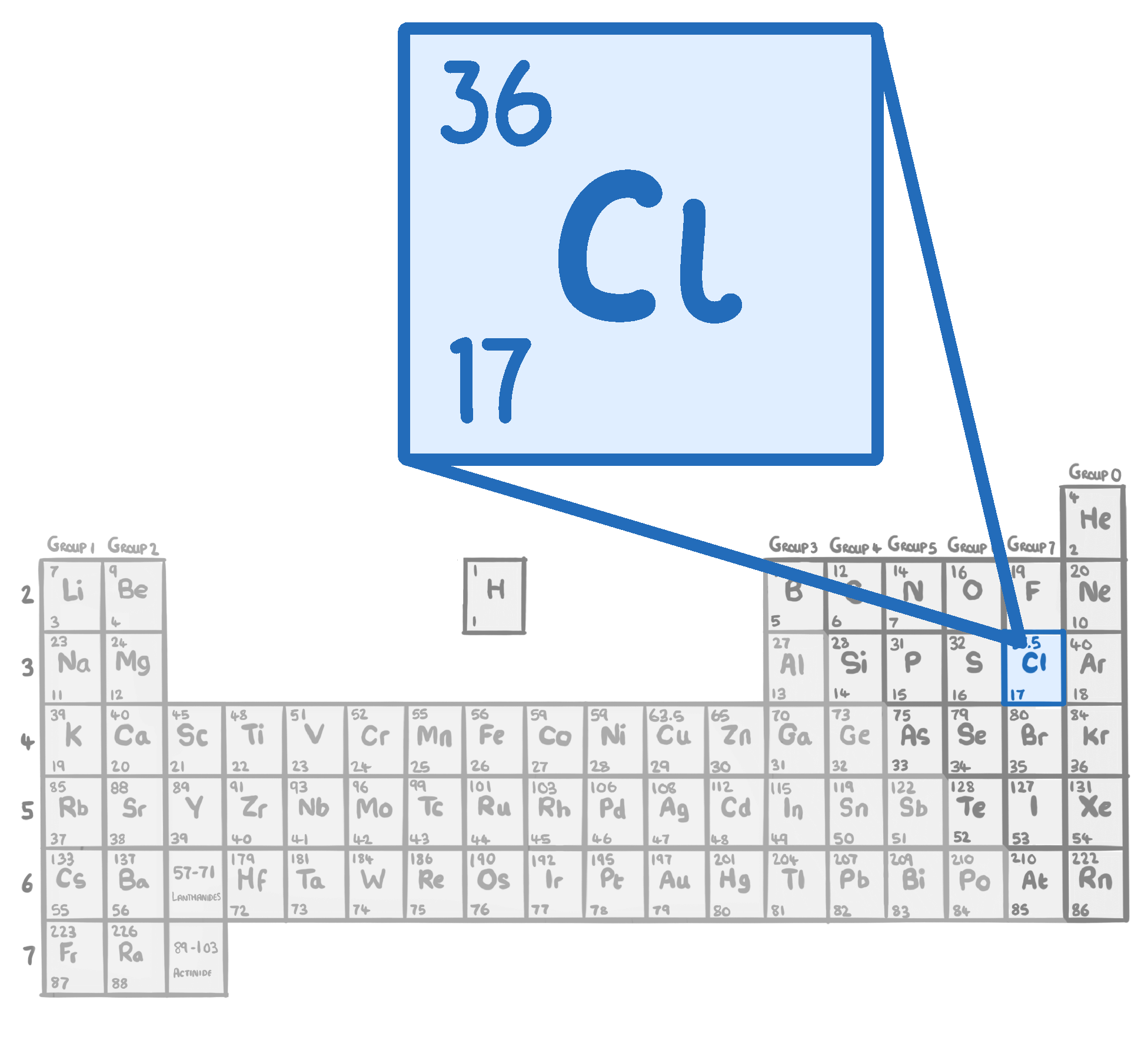
Chlorine has an atomic number of .
It's in group of the periodic table. This means it has electrons in its outer shell, and has similar chemical properties to the other elements in the same group
|
Remember the final column in the periodic table is called group 0, not group 8.
The elemental atoms in this group have full outer shells of electrons, and so are stable and unreactive.
Are there more metal or non-metal elements?
Metal
Non-metal
|
The atoms of elements in the same period have the same number of:
Protons
Electrons
Shells
Electrons in their outer shell
|