Reaction of Alkenes
This lesson covers:
- The difference between alkanes and alkenes
- What an 'addition reaction' is
- How alkenes react with hydrogen, water, and halogens
- Distinguishing between alkanes and alkenes using bromine water
Is an alkene a saturated or unsaturated hydrocarbon?
Saturated
Unsaturated
|
Which elements do alkenes contain?
(Select all that apply)
Carbon
Oxygen
Hydrogen
Nitrogen
|
What is the key difference between alkanes and alkenes?
Alkenes have a carbon to carbon double bond whereas alkanes do not
Alkenes have oxygen atoms whereas alkanes do not
Alkenes are not a homologous series
|
What type of reaction can an alkene be part of, because of the carbon to carbon double bond?
Addition reactions
Neutralisation reactions
Condensation reactions
|
Addition reaction between an alkene and hydrogen

Reacting an alkene with hydrogen gas, and a catalyst, will produce an alkane.
C3H6 + H2 ➔ C3H8
Which compound is produced in the following reaction?
C3H6 + H2 ➔ ?
Butane C4H10
Propane C3H8
Hexane C6H14
Pentane C5H12
|
Addition reaction between an alkene and water
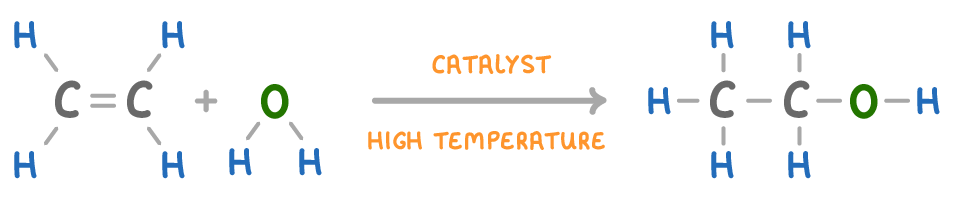
Reacting an alkene with water (at a high temperature and with a catalyst) will produce an alcohol.
C2H4 + H2O ➔ C2H5OH
Which compound is produced in the following reaction?
C2H4 + H2O ➔ ?
Propanol C3H7OH
Hexanol C6H13OH
Butanol C4H9OH
Ethanol C2H5OH
|
An addition reaction between an alkene and water will produce an alcohol.
Once produced, the alcohol will dissolve in the water.
What process can be used to separate the alcohol from the water?
Crystallisation
Grinding
Filtering
Fractional distillation
|
Addition reaction between an alkene and a halogen
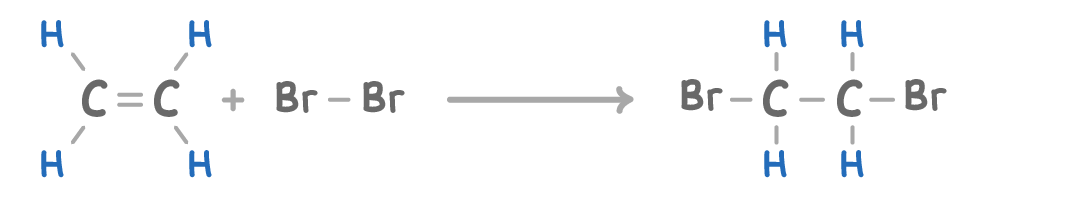
Reacting an alkene with a halogen will produce a halogenoalkane.
C2H4 + Br2 ➔ C2H4Br2
Which compound is produced in the following reaction?
C2H4 + Br2 ➔ ?
Dibromomethane CH2Br2
Dibromobutane C4H8Br2
Dibromopropane C3H6Br2
Dibromoethane C2H4Br2
|
Reacting an alkene with a halogen will produce a halogenoalkane.
C4H8 + Br2 ➔ ?
Butene (C4H8) can react with Bromine (Br2) to produce a halogenoalkane.
What halogenoalkane is produced in the reaction?
Dibromopropane C3H6Br2
Dibromobutane C4H8Br2
Dibromohexane C6H12Br2
Dibromopentane C5H10Br2
|
An unidentified liquid could be either an alkene or an alkane. To test which one it is, the liquid is reacted with bromine water.
The bromine water turns from orange to colourless.
What type of hydrocarbon is the liquid?
An alkane
An alkene
|