Cells and Batteries
This lesson covers:
- How cells (and batteries) produce electricity
- The factors that affect the voltage of a cell
- The difference between rechargeable and non-rechargeable cells
Cells |
Electrochemical cells (or 'cells'), use chemical reactions to produce electricity. |
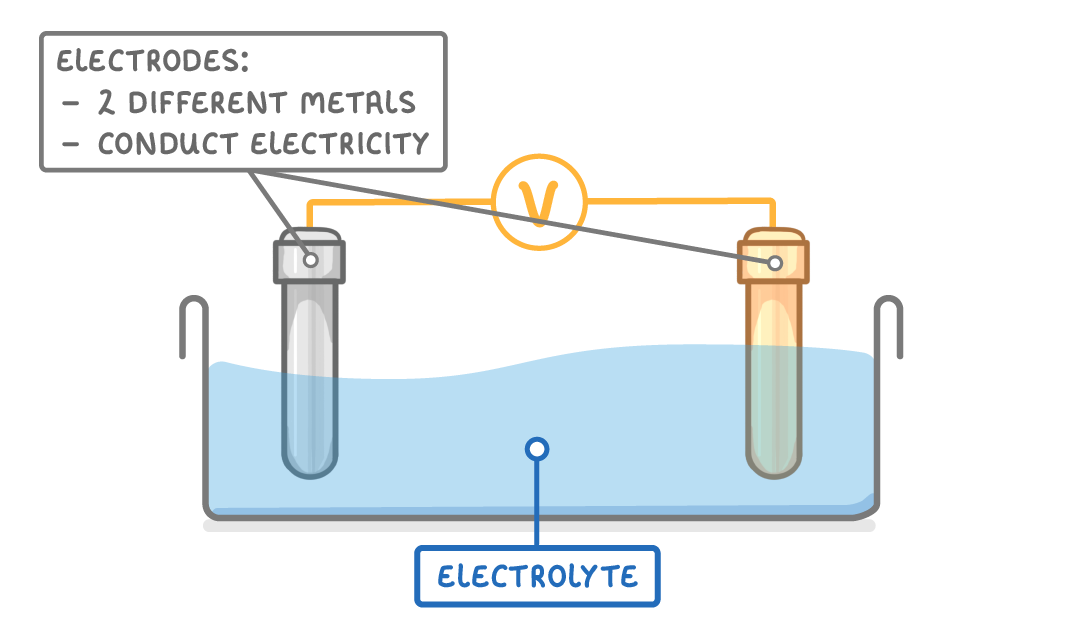 A cell can be made by connecting two different electrodes with a wire, and placing them in contact with an electrolyte. An electrolyte is just a liquid through which charged ions can flow. There will often also be a voltmeter so that you can measure the voltage of the cell. |
 |
Batteries |
 Batteries are very similar, but consist of two or more cells connected together in series to provide a greater voltage. |
Factors that affect the voltage of a cell
- The metals used for the two electrodes. The greater the difference in reactivity of the two metals, the greater the voltage will be.
- The type and concentration of the electrolyte used.
- The conditions, such as temperature.
Rechargeable vs non-rechargeable cells |
Rechargeable cells and batteries can be recharged because the chemical reactions are reversed when an external electrical current is supplied. These are the batteries used in laptops and mobile phones. |
In non-rechargeable cells and batteries the chemical reactions stop when one of the reactants has been used up. These are the batteries usually used in smoke alarms and tv remotes. Alkaline batteries are non-rechargeable. |
What is the name of the liquid that conducts electricity in electrochemical cells?
|
How do chemical cells transfer energy to their surroundings?
By heating
By light
By electricity
|

What do we call the pieces of metal in an electrochemical cell?
|
What word is used to describe multiple electrochemical cells connected together, that results in increased voltage?
|

Which type of battery is likely to be found in wireless headphones?
Rechargeable battery
Non-rechargeable battery
|
Are alkaline batteries rechargeable or non-rechargeable?
Non-rechargeable
Rechargeable
|