The Reactivity Series & Displacement Reactions
This lesson covers:
- The reactions of metals with acid and water
- The features of the reactivity series
- The mechanism of displacement reactions
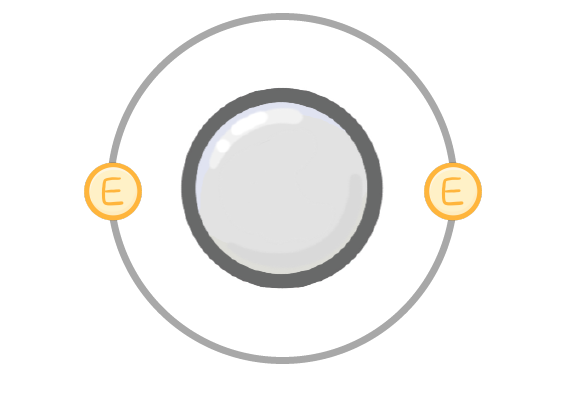
Which factor determines the reactivity of a metal?
How easily the atoms of that element gain electrons
How easily the atoms of that element lose their outer electrons
|
Which group of metals are the most reactive?
Transition metals
Group 2
Group 1
Group 3
|
When metals react, do the atoms become positive or negative ions?
Positive
Negative
|
Do metals react more violently with water or acid?
Water
Acid
|
The general word equation for the reaction between a metal and an acid is:
Metal + acid ➔ salt +
|
The word equation for the reaction between magnesium and hydrochloric acid is:
Magnesium + hydrochloric acid ➔ + hydrogen
|
The general word equation for the reaction between a metal and an acid is:
Metal + acid ➔ ____________ + hydrogen
water
metal oxide
salt
|
When potassium reacts with water, the metal whizzes around on the surface, and the hydrogen gas catches fire violently.
When magnesium reacts with water, bubbles form on the surface but there is no flame.
Which metal is more reactive?
Potassium
Magnesium
|

Sodium reacts with water to produce sodium hydroxide and a gas.
What is the name of the gas produced?
Oxygen
Hydrogen
Carbon dioxide
Water vapour
|
Which of the following is the correct description of displacement reactions?
A more reactive metal displaces a less reactive metal
A less reactive metal displaces a more reactive metal
|
Magnesium (Mg) is reacted with copper sulfate (CuSO4).
Which of the following is the correct equation for the reaction?
MgSO4 + CuSO4 ➔ Cu + Mg + SO4
Mg + CuSO4 ➔ MgSO4 + Cu
Mg + CuSO4 ➔ Mg + H2O
|
What would happen if iron and lithium sulfate were mixed together?
Nothing
The iron would displace the lithium
|