Acids and alkalis
This lesson covers:
- The pH scale
- Using indicators
- Comparing acid and alkali strength
The pH Scale
The pH scale measures the acidity or alkalinity of a solution on a scale from 0 to 14.
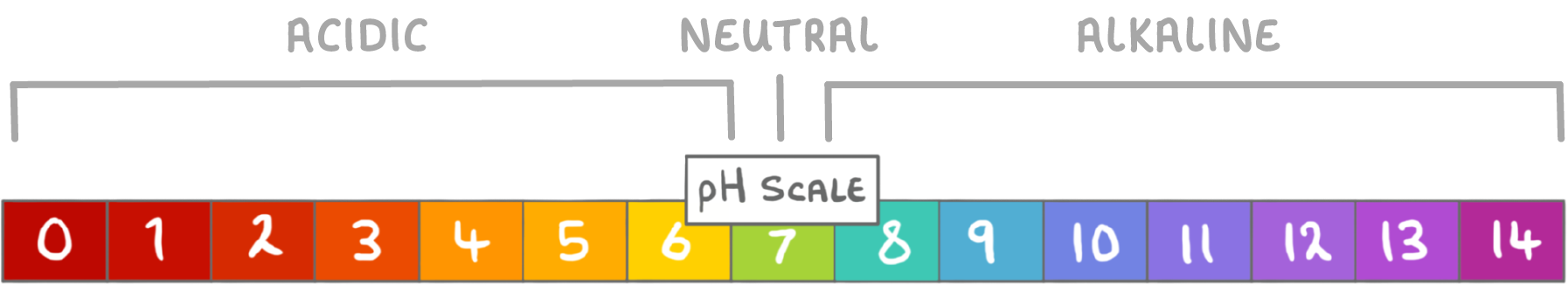
- pH 7 is neutral.
- Below pH 7 is acidic .
- Above pH 7 is alkaline.
Using Indicators
Indicators are special dyes that change colour depending on the pH.
Litmus paper and universal indicator are two common indicators.
Litmus paper as an indicator
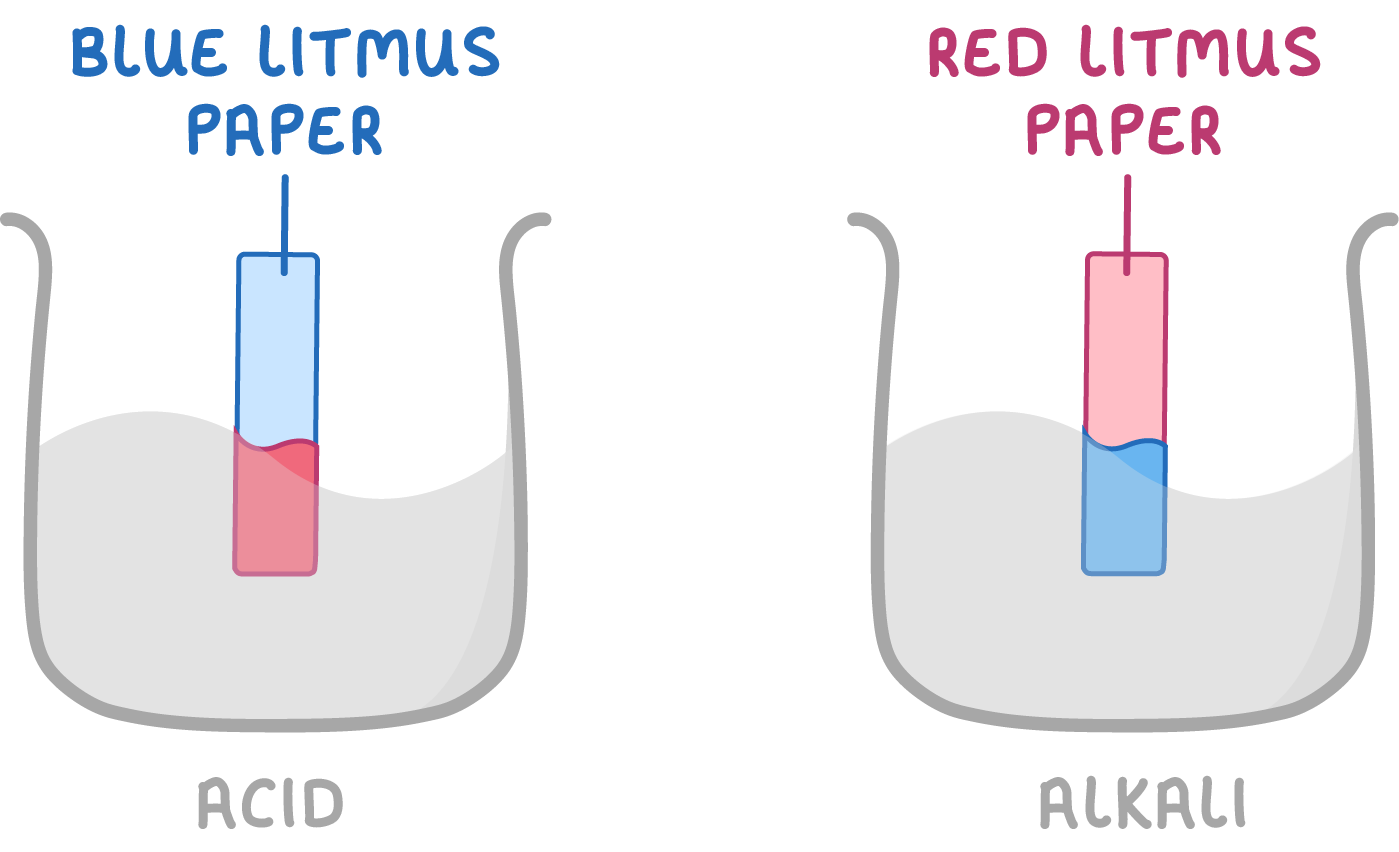
- Blue litmus paper turns red in acids.
- Red litmus paper turns blue in alkalis.
Universal indicator
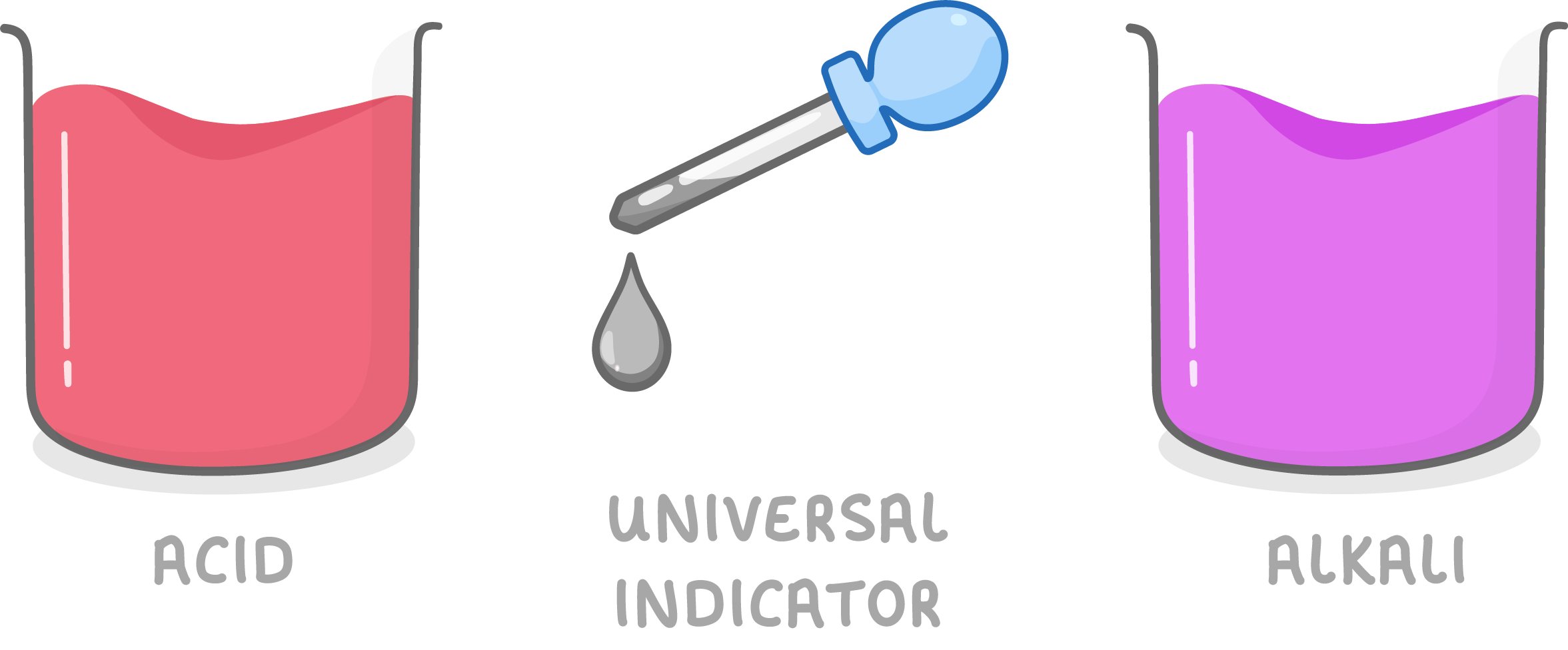
Universal indicator solution can be added to substances.
- It contains a mixture of indicators that change across pH range.
- The colours match up with different pH levels on the pH scale, telling us if a substance is acid, alkali or neutral.
Acid and Alkali Strength
The pH scale also shows us the relative strength of acids and alkalis.
- The lower the pH, the stronger the acid.
- The higher the pH, the stronger the alkali.
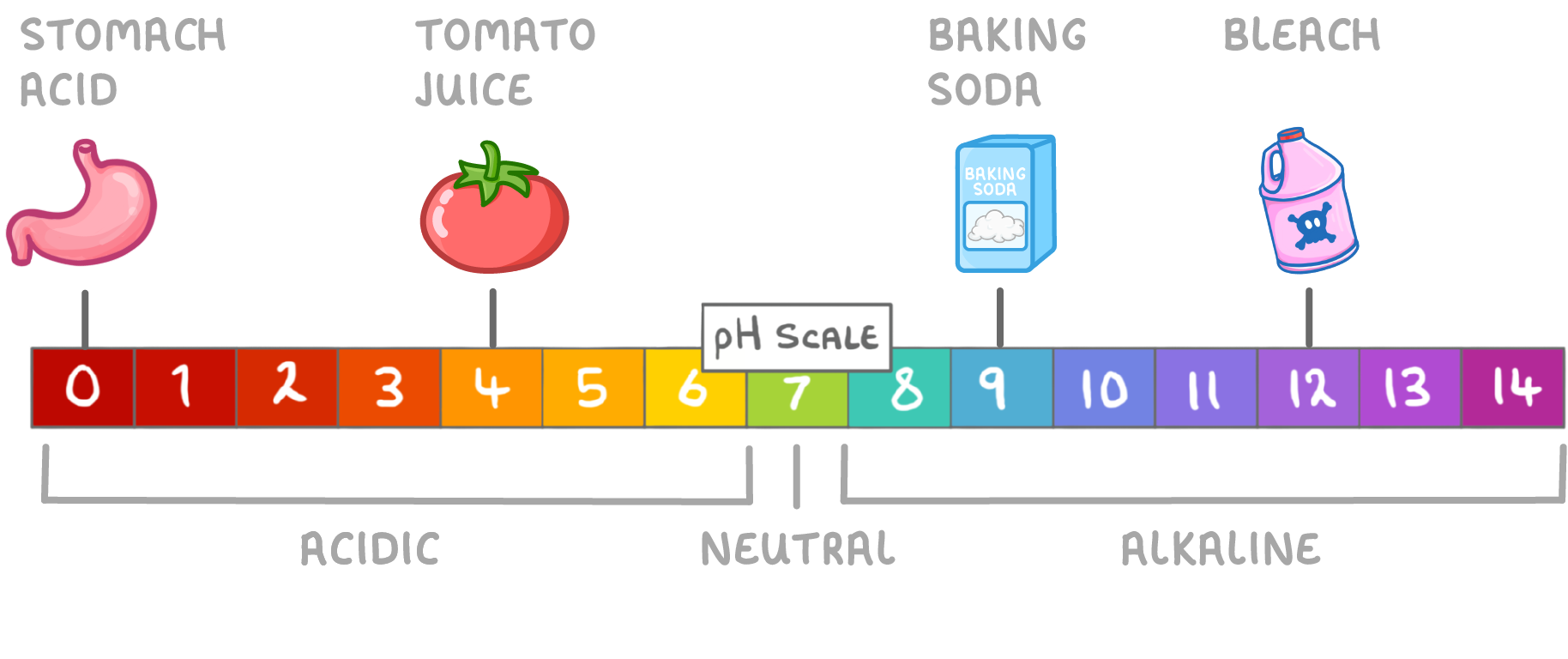
For example:
- Stomach acid = pH1, so it is a strong acid.
- Tomato juice = pH 4 , so it is a weak acid.
- Baking soda = pH 9, so it is a weak alkali.
- Bleach = pH 12, so it is a strong acid.