Balancing equations
This lesson covers:
- Ways to show chemical equations
- How to balance symbol equations
Chemical equations
Chemical reactions can be represented by word and symbol equations.
Types of equation
- Word equations - Use the names of reactants and products.
- Symbol equations - Use chemical formulas and symbols.
- Balanced symbol equations - Show the number of reactants and products in a reaction.
Examples:
- Word equation - magnesium + oxygen —> magnesium oxide
- Symbol equation - Mg + O2 —> MgO
- Balanced symbol equation - 2Mg + O2 —> 2MgO
Balancing symbol equations
To balance a symbol equation follow these steps:
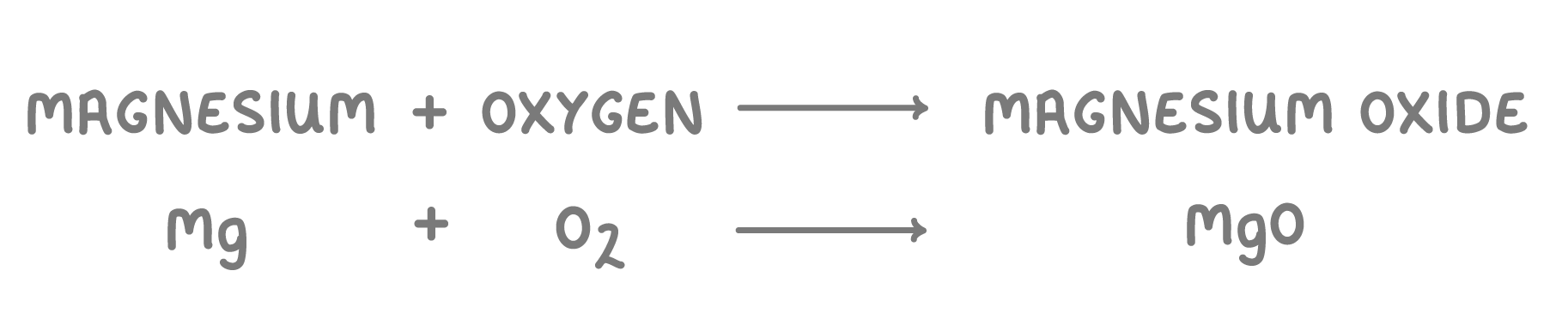
1Write the word equation for the reaction.
2Then add in the chemical formulae to form a symbol equation
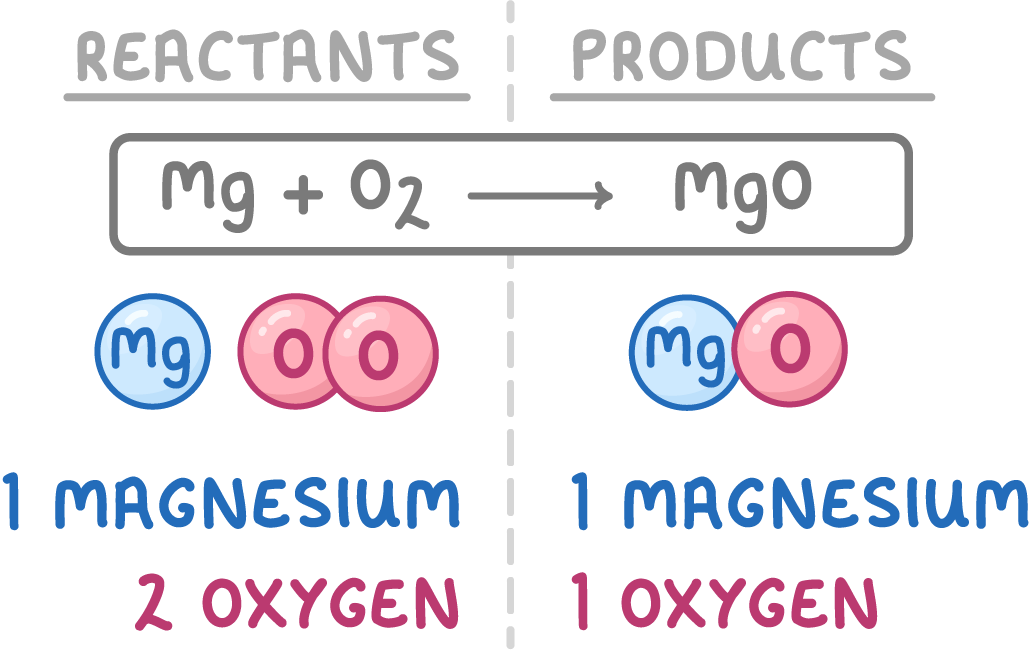
3Count the numbers of each type of atom on both sides of the equation.
4This equation is unbalanced as there are more oxygen atoms on the left hand side than the right hand side.
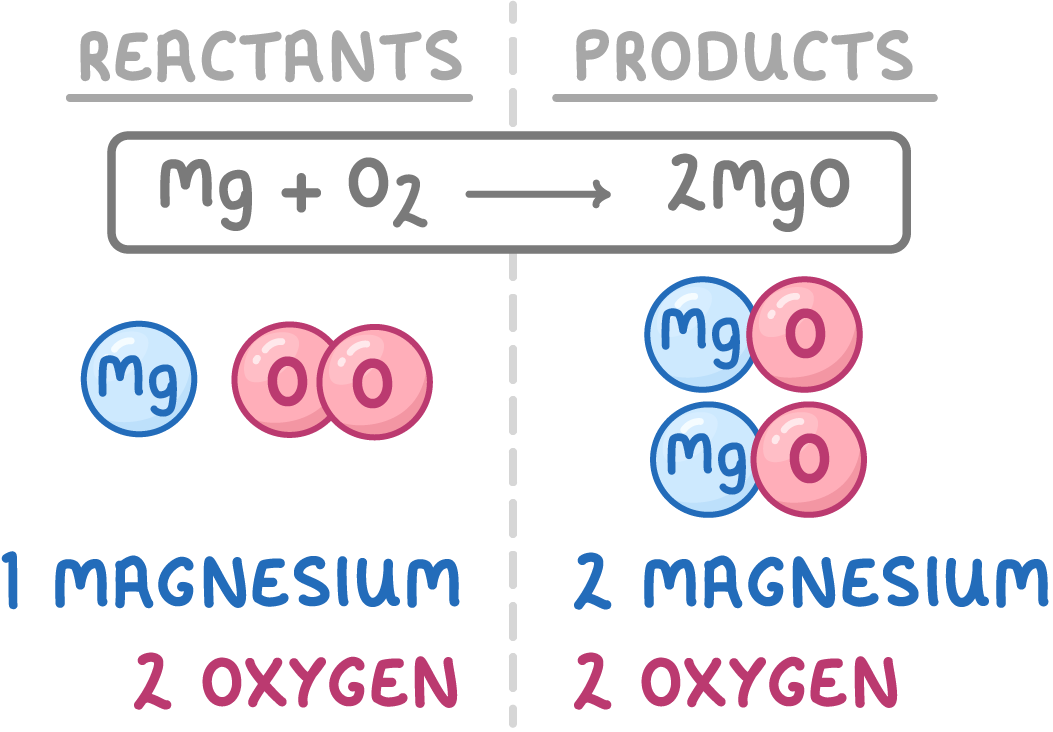
5Pencil in numbers before formulas to try to balance unequal atoms.
6Count the numbers of each type of atom on both sides of the equation.
7This equation is still unbalanced as there are more magnesium atoms on the right hand side than the left hand side.
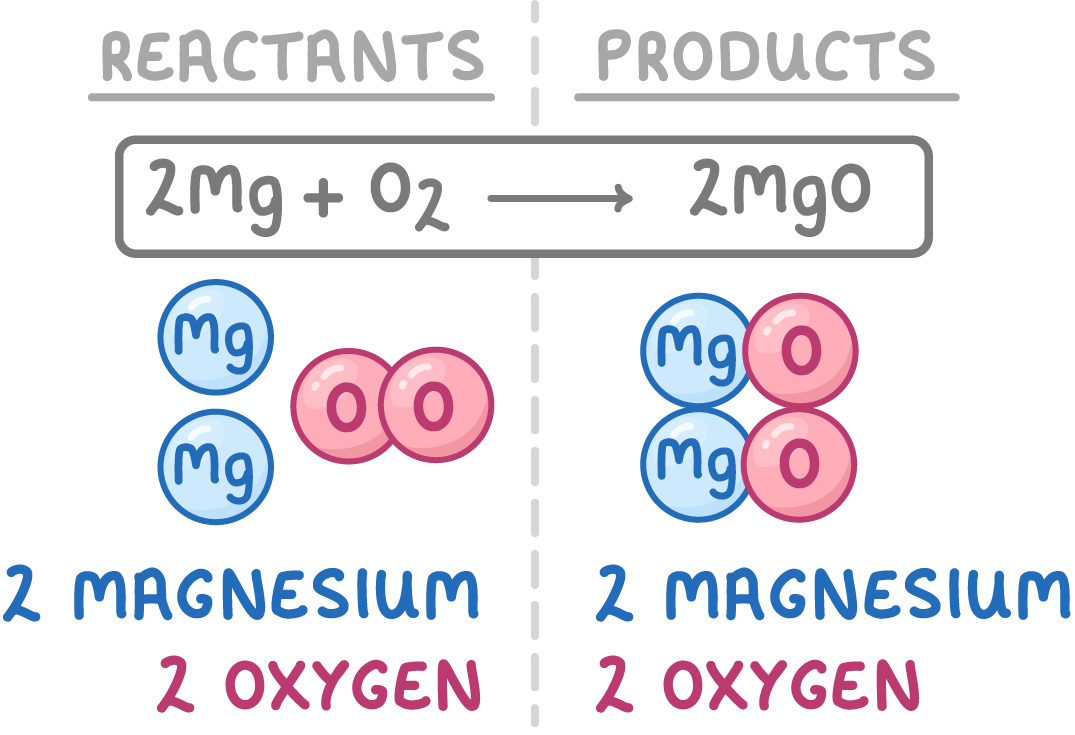
8We repeat the steps 5 and 6 until the numbers of each type of atom are the same on both sides of the arrow.
9This gives us the balanced symbol equation for this chemical reaction.